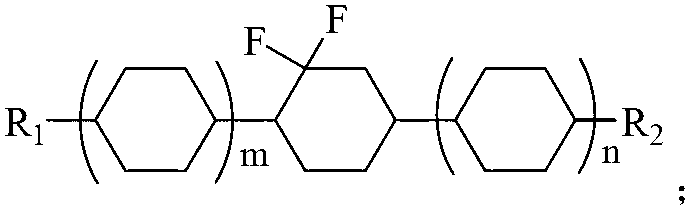
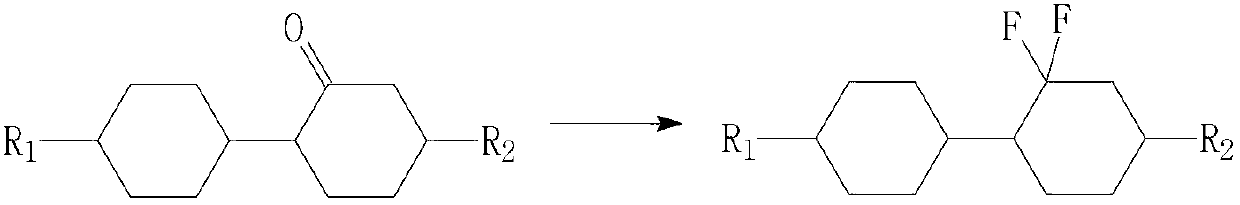
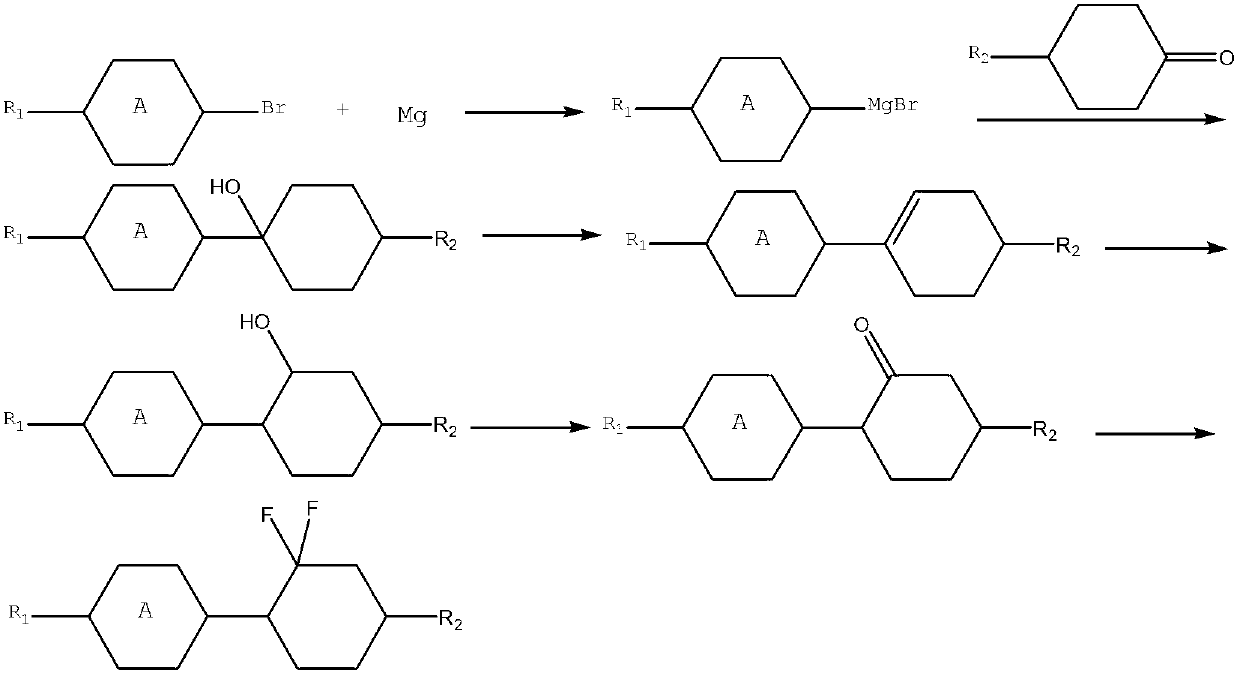
Preparation method of difluorocyclohexane liquid crystal compound
A technology of liquid crystal compounds and difluorocyclohexane, which is applied in the field of synthesis of liquid crystal compounds, can solve the problems of unrecorded preparation methods of intermediate ketones, cumbersome preparation methods, and low reaction yields, and achieve low cost, simple process, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The reaction scheme for preparing difluorocyclohexane compounds in this example is as follows:
[0049]
[0050] Compound E is prepared according to the following steps:
[0051] (1) Add 160ml of dichloromethane and 3.75mol of iodine into a 1L three-neck flask. After the iodine is fully dissolved, under the protection of nitrogen, cool down to -10°C, add 3.75mol of pyridine dropwise, stir and mix evenly, and then control the temperature- Add 3.75 mol of triphenylphosphine in batches at 10°C, stir for 30 minutes, then add 0.25 mol of compound F dropwise under temperature control at -10°C, and naturally rise to room temperature for 10 hours to obtain compound G;
[0052](2) Add 900ml of ethanol into a 2L three-neck flask, add 10mol of sodium under stirring, stir until completely dissolved, add 5mol of ethyl acetoacetate dropwise at 50°C under temperature control, stir for 30min, add 1mol of compound G dropwise, and react at 50°C for 2h , to obtain compound A;
[0053...
Embodiment 2
[0060] The reaction scheme for preparing difluorocyclohexane compounds in this example is as follows:
[0061]
[0062] Compound E is prepared according to the following steps:
[0063] (1) Add 440ml of benzene and 2.5mol of iodine to a 1L three-necked flask. After the iodine is fully dissolved, under the protection of nitrogen, cool down to 0°C, add 1.25mol of pyridine dropwise, stir and mix evenly, and then control the temperature at 0°C in batches Add 1.25 mol of triphenylphosphine, stir for 30 minutes, then add 0.25 mol of compound F dropwise under temperature control at 0°C, let it naturally rise to room temperature and react for 14 hours to obtain compound G;
[0064] (2) Add 580ml of ethanol into a 2L three-neck flask, add 5mol of sodium under stirring, stir until completely dissolved, add 10mol of ethyl acetoacetate dropwise at 60°C under temperature control, stir for 40min, add 1mol of compound G dropwise, and react at 60°C for 1h , to obtain compound A;
[0065]...
Embodiment 3
[0072] The reaction scheme for preparing difluorocyclohexane compounds in this example is as follows:
[0073]
[0074] Compound E is prepared according to the following steps:
[0075] (1) Add 380ml of dichloromethane and 1.25mol of iodine to a 1L three-neck flask. After the iodine is fully dissolved, under the protection of nitrogen, cool down to -5°C, add 1.25mol of pyridine dropwise, stir well, and then control the temperature to -5°C. Add 1.25 mol of triphenylphosphine in batches at ℃, stir for 30 minutes, then add 0.25 mol of compound F dropwise under temperature control at -5 ℃, let it naturally rise to room temperature and react for 12 hours to obtain compound G;
[0076] (2) Add 1200ml of ethanol to a 2L three-neck flask, add 2mol of sodium under stirring, stir until completely dissolved, add 2mol of ethyl acetoacetate dropwise at 55°C under temperature control, stir for 50min, add 1mol of compound G dropwise, and react at 55°C for 2h , to obtain compound A;
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com