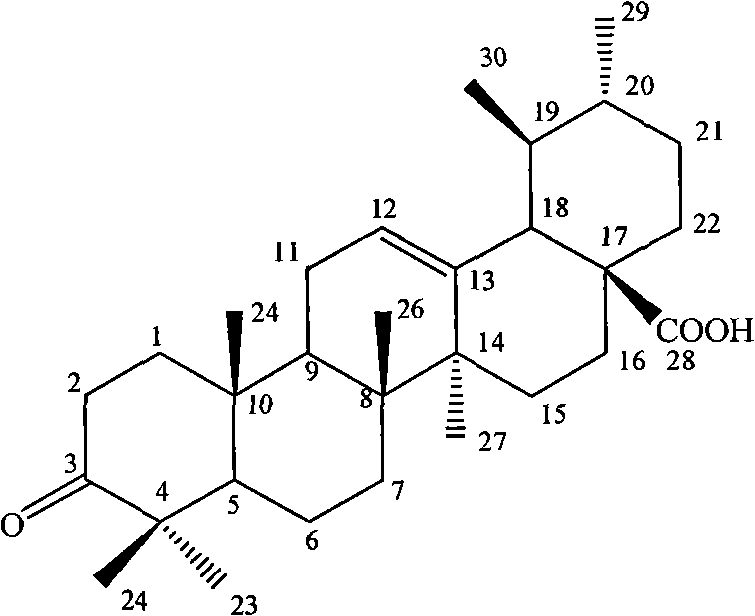
Method for preparing ursolic acid derivative 3-o-keto-12-alkenyl-28-ursolic acid
A technology of ursolic acid and ethyl acetate, applied in the field of natural medicine, can solve the problems of difficult separation of product and catalyst, low yield, and total yield of only 80%, and achieves rapid separation, high product purity, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] ① Dissolve 1.11g of ursolic acid in 10ml of dry dichloromethane, cool in an ice bath, then add 0.6g of pyridinium chlorochromate, stir and react for 4 hours, remove the ice bath, and react at room temperature for 8 hours.
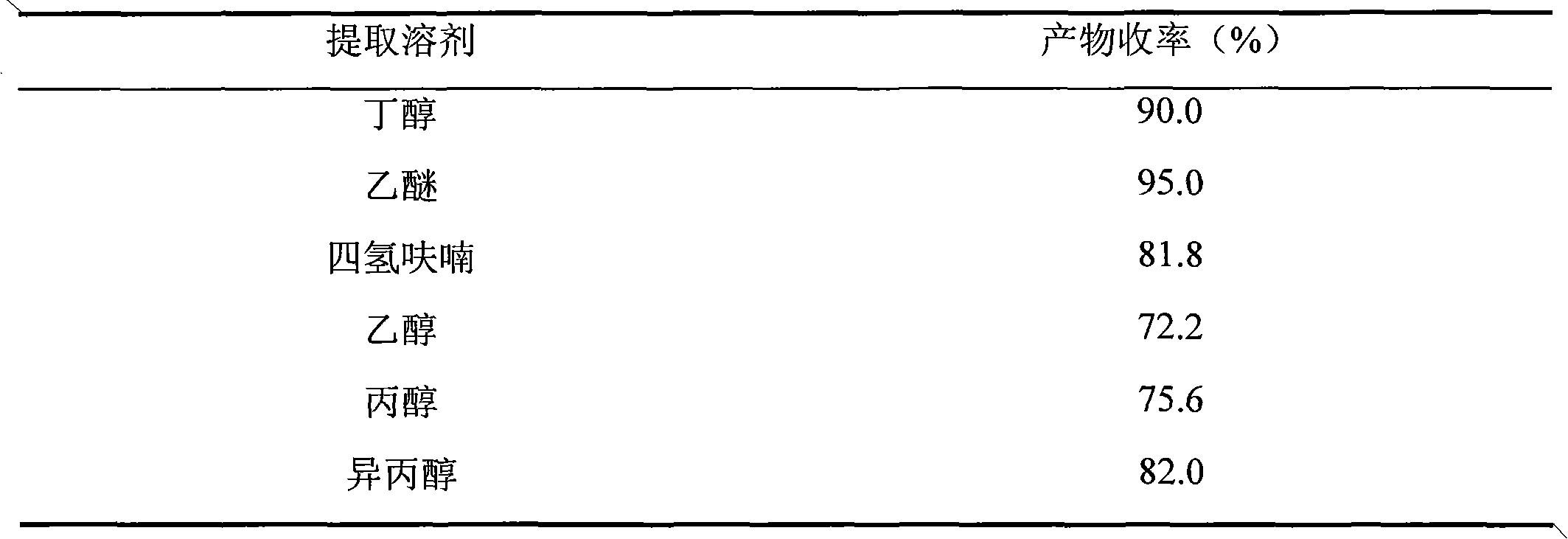
[0021] ② Concentrate and dry the above-mentioned reaction solution under reduced pressure, then extract the product with butanol 3 times, 10ml each time, filter, combine the organic phases, and concentrate under reduced pressure at 30°C to obtain 3-O-keto-12-ene-28 - Crude ursolic acid 1.12g.
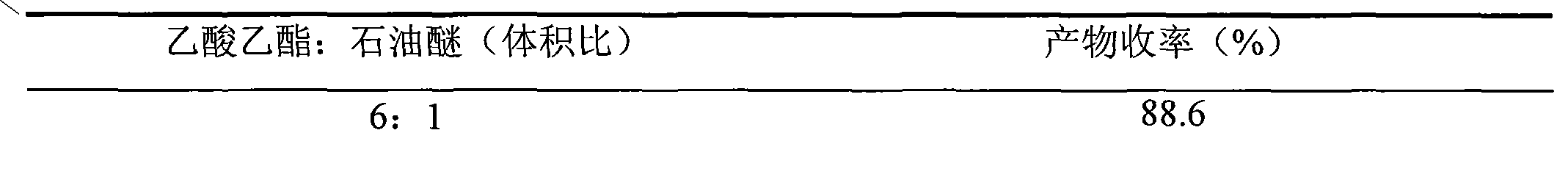
[0022] ③Separate the crude 3-O-keto-12-ene-28-ursolic acid through a column with 10 g of 300-400 mesh silica gel, apply a dry method to the column, and use ethyl acetate to petroleum ether = 5:1 as the eluent , 1.00 g of pure product was obtained, the yield was 90.0%, and the purity was 99.0% (HPLC test, area normalization method).
[0023] The physical and chemical properties of the isolated 3-O-keto-12-ene-28-ursolic acid are as follows:
[0024] Melting p...
Embodiment 2
[0031] ① Dissolve 22.0g of ursolic acid in 180.0ml of dry dichloromethane, cool in an ice bath, then add 12.5g of pyridinium chlorochromate, stir and react for 4 hours, remove the ice bath, and react overnight at room temperature.
[0032] ②Concentrate the reaction liquid after the above reaction to dryness under reduced pressure, then extract the product with ether 5 times, 30ml each time, filter, combine the organic phases, and concentrate under reduced pressure at 55°C to obtain 3-O-keto-12-ene-28- Crude ursolic acid 21.8g.
[0033] ③Separate the crude 3-O-keto-12-ene-28-ursolic acid through a column with 100 g of 300-400 mesh silica gel, put it on the column by dry method, and use ethyl acetate to petroleum ether = 3:1 as the eluent , 20.9 g of pure product was obtained, the yield was 95.0%, and the purity was 99.0% (HPLC test, area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com