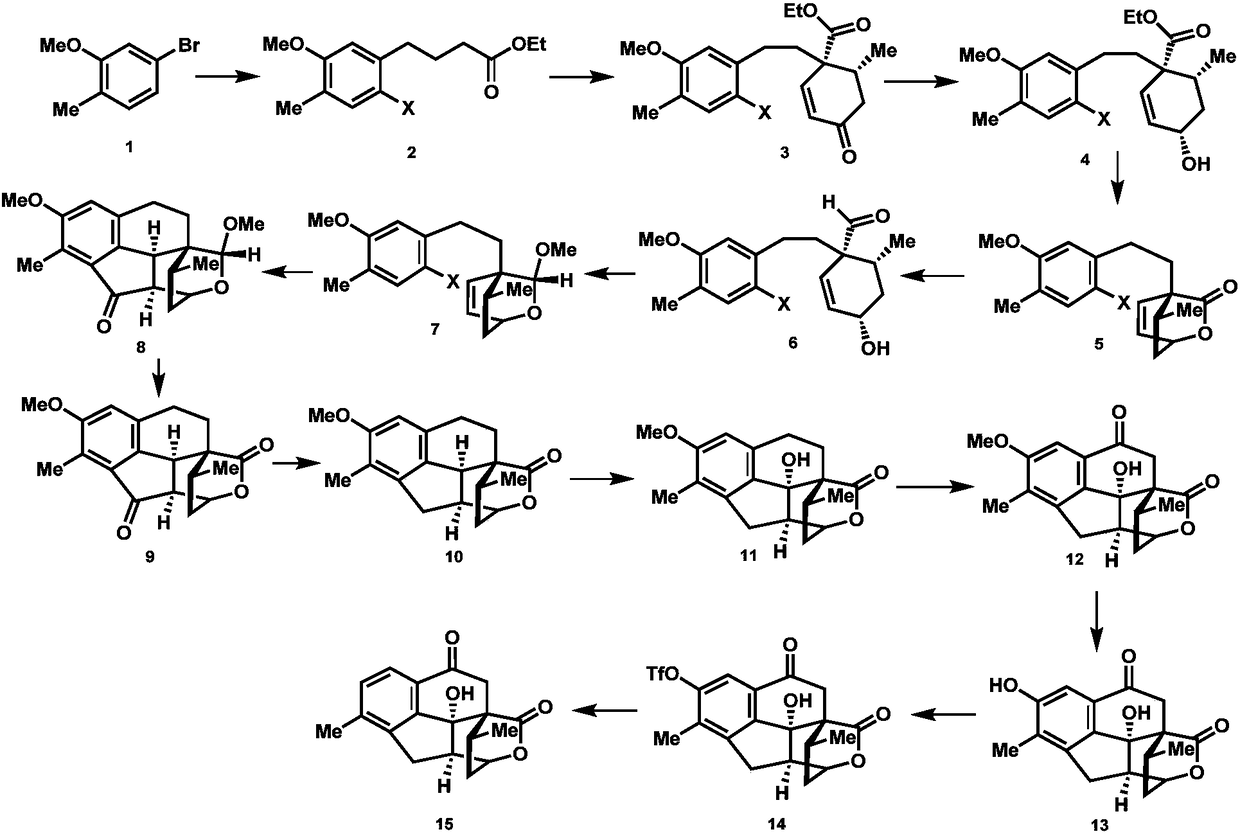
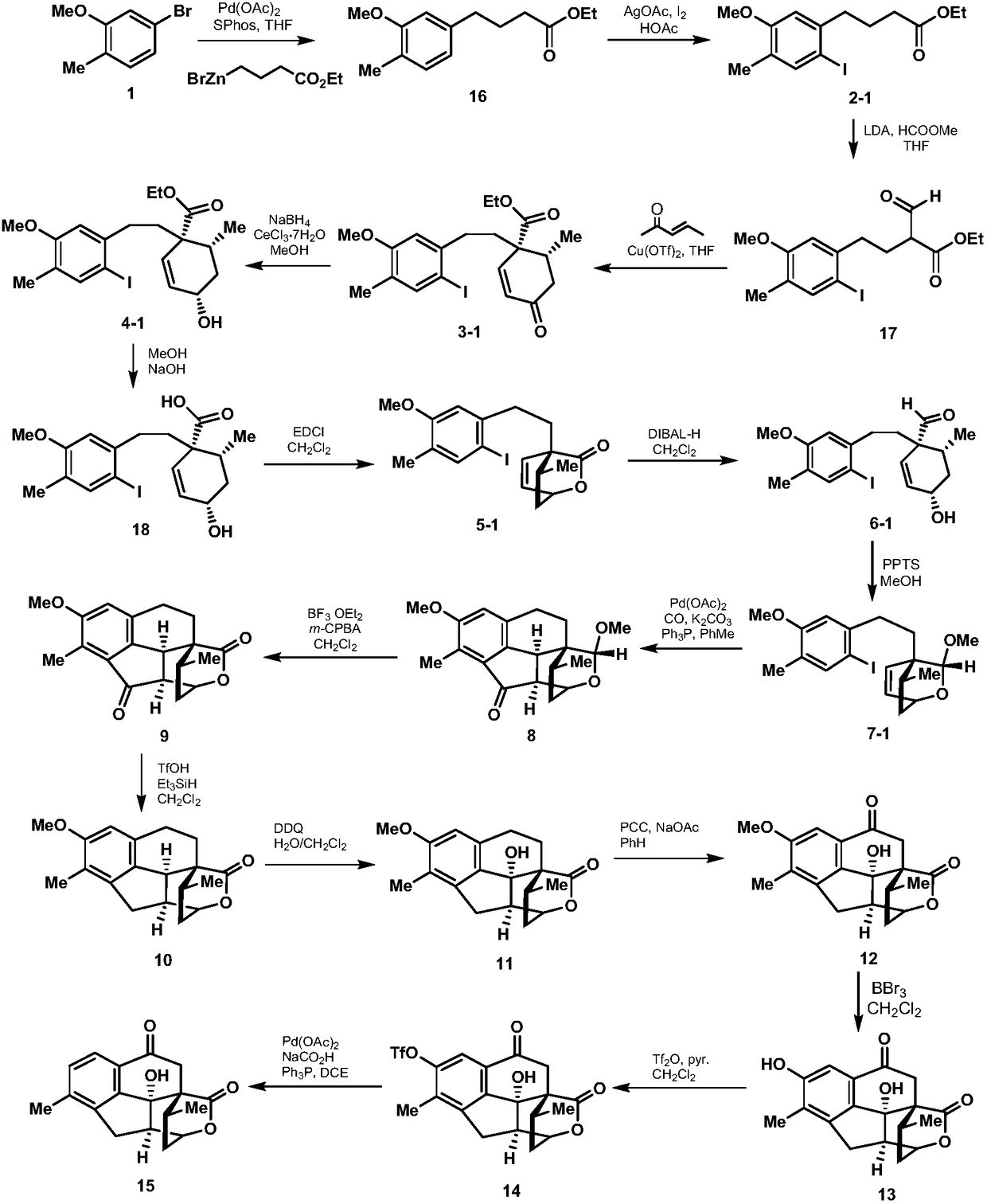
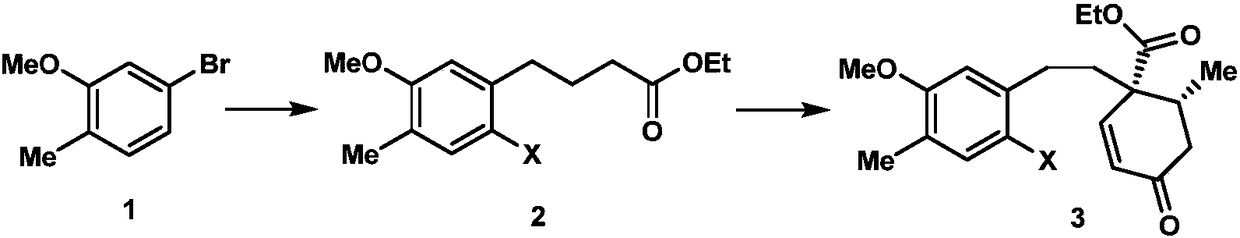
Synthesis method of cephanolide C
A synthetic method and compound technology, which is applied in the field of natural product synthesis, can solve the problems of chemical synthesis method reporting, etc., and achieve the effect of low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] 1. Add 24.1g (120mmol) of 5-bromo-2-methylanisole shown in formula 1, 539mg (2.4mmol) of palladium acetate, 1.1g (4.8mmol) of S-Phos in a 500mL dry round bottom flask , then add 50mL of degassed tetrahydrofuran, stir at room temperature for 5 minutes under the protection of nitrogen, then add 300mL of 0.6mol / L tetrahydrofuran solution of ethyl 4-bromobutyrate zinc reagent, stir at 50°C for 5 hours, then mix with ethyl acetate and saturated The ammonium chloride aqueous solution was extracted, the organic phase was collected, dried over sodium sulfate, spin-dried, and passed through the column with petroleum ether and ethyl acetate to obtain 27.8 g of the compound shown in formula 16, with a yield of 98%. The structural characterization data are as follows: 1 H NMR (600MHz, deuterated chloroform) δ7.07(d, J=7.5Hz, 1H), 6.71(d, J=7.5Hz, 1H), 6.69(s, 1H), 4.16(q, J=7.6, 7.2Hz, 2H), 3.85(s, 3H), 2.66(t, J=7.7Hz, 2H), 2.36(t, J=7.5Hz, 2H), 2.23(s, 3H), 1.99(p, J=...
Embodiment 2
[0042] In step 2 of Example 1, the 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride used is replaced with equimolar dicyclohexylcarbodiimide, other steps of this step Same as Example 1, the lactone compound represented by formula 5-1 was obtained, and the total yield of the two steps was 57%. Other steps are identical with embodiment 1.
Embodiment 3
[0044] In step 3 of Example 1, the used p-toluenesulfonic acid pyridinium salt is replaced with equimolar p-toluenesulfonic acid, other steps of this step are the same as in Example 1, and the acetal compound shown in formula 7-1 is obtained, and the yield is 58%. Other steps are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com