2-tertbutyloxycarbonyl-7-carbonyl-5-O-2-azaspiro(3.4)octane synthesis method
A technology of tert-butoxycarbonyl and synthesis method, which is applied in the field of synthesis of 2-tert-butoxycarbonyl-7-carbonyl-5-oxo-2-azaspiro(3.4) octane, which can solve the problem of expensive reagents and expensive , high risk and other problems, to achieve the effect of reasonable reaction process design, saving synthesis cost, and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] Slowly add 130 grams of sodium bisulfite aqueous solution to 120 grams of compound 2 and 130 g of sodium periodate in 2.8 liters of acetonitrile aqueous solution (volume ratio, 3:1), and the reaction solution was slowly raised to 25° C. to react overnight. compound 2 If the reaction is not complete, add saturated sodium thiosulfate solution to quench the reaction, extract with ethyl acetate, wash the organic layer with water and add anhydrous sodium sulfate to dry, filter, and rotary evaporate under reduced pressure to obtain a yellow oil, and obtain a crude product, column chromatography Isolation and Purification. Yield: 20%.
[0017] 1 H-NMR (CDCl3): 4.10-3.90 (m, 6H), 3.60 (m, 1H), 5.19-5.18 (m, 1H), 2.10-1.81 (m, 2H), 1.43 (s, 9H).
Embodiment 2
[0019]
[0020] Slowly add 130 grams of sodium bisulfite aqueous solution to 120 grams of compound 2 and 130 grams of sodium periodate in 2.8 liters of acetonitrile aqueous solution (volume ratio, 3:1), and the reaction solution was slowly raised to 50° C. to react overnight. After the reaction was completed, saturated sodium thiosulfate solution was added to quench the reaction, extracted with ethyl acetate, the organic layer was washed with water and dried by adding anhydrous sodium sulfate, filtered, and rotary evaporated under reduced pressure to obtain a yellow oil, and the crude product was separated by column chromatography. purification. Yield: 42%.
Embodiment 3
[0022]
[0023] Slowly add 130 grams of sodium bisulfite aqueous solution to 120 grams of compound 2 and 130 g of sodium periodate in 2.8 liters of acetonitrile aqueous solution (volume ratio, 3:1), and the reaction solution was slowly raised to 80° C. to react overnight. After the reaction was completed, saturated sodium thiosulfate solution was added to quench the reaction, extracted with ethyl acetate, the organic layer was washed with water and dried by adding anhydrous sodium sulfate, filtered, and rotary evaporated under reduced pressure to obtain a yellow oil, and the crude product was separated by column chromatography. purification. Yield: 60%.
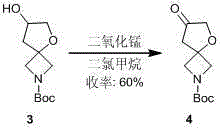
[0024] Synthesis of tert-butoxycarbonyl-7-carbonyl-5-oxo-2-azaspiro(3.4)octane
[0025] Example 1
[0026]
[0027] 44 g compound 3 It was dissolved in 400 ml of dichloromethane solution, slowly added dropwise to a mixture of 50 g of manganese dioxide and 400 ml of dichloromethane under ice-cooling, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com