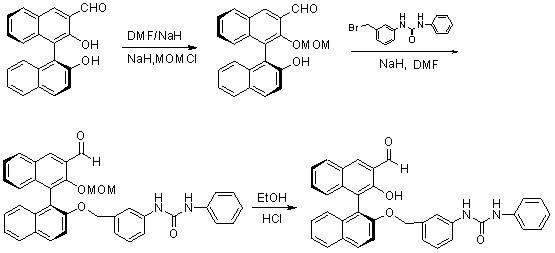
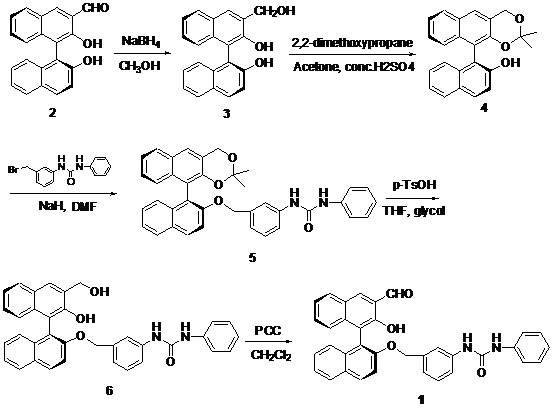
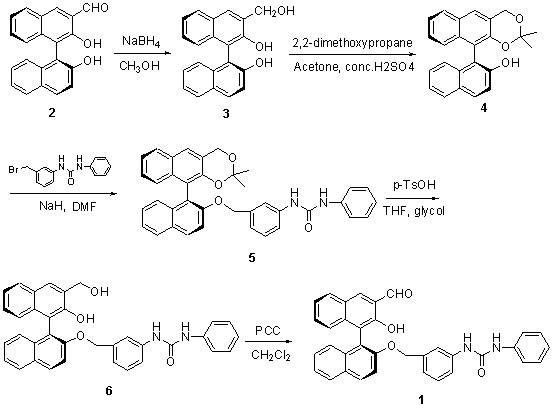
Method for synthesizing (S)-2-hydroxy-2'-(3-phenylureaphenyl)-1,1'-binaphthyl-3-formaldehyde
A technology of phenylureabenzyl and phenylureidobenzyloxy is applied in the field of synthesizing -2-hydroxy-2'--1,1'-binaphthyl-3-formaldehyde, and can solve the problem of being unsuitable for industrialized large-scale production , poor regioselectivity, complex operation, etc., to avoid the formation of complex by-products, easy to obtain, and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Take 3-formyl-1,1'-binaphthol (2) as raw material, dissolve it in a certain amount of methanol, cool in an ice bath to 10°C, add sodium borohydride, 3-formyl-1,1'- The molar ratio of binaphthol to sodium borohydride is 1.0:1.0, and the reduction reaction is carried out at room temperature to obtain 3-hydroxymethyl-1,1'-binaphthol (3); sulfuric acid) condition, the molar ratio of concentrated sulfuric acid to 3-hydroxymethyl-1,1'-binaphthol (3) is 0.01:1, add 2,2'-dimethoxypropane, 3- The molar ratio of hydroxymethyl-1,1'-binaphthol (3) to 2,2'-dimethoxypropane is 1.0:2.0. After the reaction at room temperature, it is extracted with an organic solvent (ethyl acetate), concentrated , separated by column chromatography to obtain the product ( S )-2-hydroxyl-1-(2,2,-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)-naphthalene (4), yield 85 %; Then use dry DMF as solvent, add sodium hydride under ice-cooling, add 3-(4-methoxyphenyl)-ureido-benzyl bromide after one hour ...
Embodiment 2
[0019] Take 3-formyl-1,1'-binaphthol (2) as raw material, dissolve it in methanol, cool in an ice bath to 5°C, add sodium borohydride, 3-formyl-1,1'-bis The molar ratio of naphthol to sodium borohydride is 1.0:1.5, and the reduction reaction is carried out at room temperature to obtain 3-hydroxymethyl-1,1'-binaphthol (3); then use acetone as a solvent in the catalyst (concentrated sulfuric acid ) conditions, the molar ratio of concentrated sulfuric acid to 3-hydroxymethyl-1,1'-binaphthol (3) is 0.05:1, adding 2,2'-dimethoxypropane, 3-hydroxymethyl The molar ratio of base-1,1'-binaphthol (3) to 2,2'-dimethoxypropane is 1.0:3.0. After reaction at room temperature, it is extracted with organic solvent (ethyl acetate), concentrated, and column layer The product was obtained by analysis and separation ( S )-2-hydroxyl-1-(2,2,-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)-naphthalene (4), yield 87 %; then use dry DMF as a solvent, add sodium hydride under ice-cooling, add 3-...
Embodiment 3
[0021]Take 3-formyl-1,1'-binaphthol (2) as the raw material, dissolve it in methanol, cool in an ice bath to 0°C, add sodium borohydride, 3-formyl-1,1'-bis The molar ratio of naphthol to sodium borohydride is 1.0:2.0, and the reduction reaction is carried out at room temperature to obtain 3-hydroxymethyl-1,1'-binaphthol (3); then use acetone as a solvent in the catalyst (concentrated sulfuric acid ) conditions, the molar ratio of concentrated sulfuric acid to 3-hydroxymethyl-1,1'-binaphthol (3) is 0.03:1, add 2,2'-dimethoxypropane, 3-hydroxymethyl-1 , the molar ratio of 1'-binaphthol (3) to 2,2'-dimethoxypropane is 1.0: 4.0, after reaction at room temperature, it is extracted with an organic solvent (ethyl acetate), concentrated, and separated by column chromatography to obtain product( S )-2-Hydroxy-1-(2,2,-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)-naphthalene (4), yield 90 %; Then use dry DMF as solvent, add sodium hydride under ice-cooling, add 3-(4-methoxypheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com