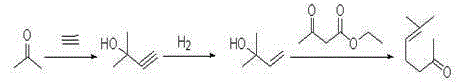
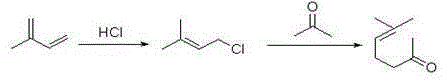Method for preparing methyl heptenone by using 3-methylcrotonaldehyde
A technology of methyl heptenone and prenyl aldehyde, which is applied in the field of preparation of methyl heptenone, can solve the problems of complicated separation and purification and raw material recovery process, equipment corrosion, large environmental pollution, and low conversion rate of raw materials, and achieves The effect of low production cost, low price and high molar yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 A method for preparing methyl heptenone by isopentenal
[0048] condensation step
[0049] (1) Preparation of solid catalyst
[0050] Using γ-alumina as a carrier, NaOH / Al with a mass percentage of NaOH of 10% was prepared by equal volume impregnation method 2 o 3 Solid base catalyst, dried in an oven at 100-120°C for 24 hours, and roasted in a muffle furnace at 500°C for 3 hours.
[0051] (2) Add acetone and catalyst
[0052] Add 150.0g of acetone to a 1000ml three-neck flask with mechanical stirring and a condenser, and add 10g of NaOH with a mass percentage of 10% NaOH / Al 2 o 3 Solid base catalyst, mechanical stirring.
[0053] (3) Drop prenaldehyde
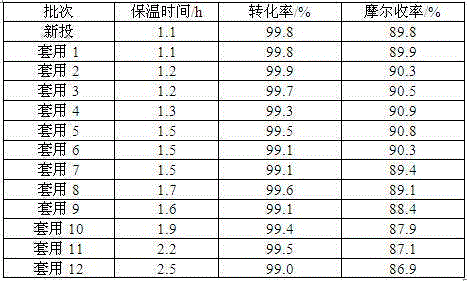
[0054]Add 110.0 g prenaldehyde dropwise at 50°C for 1 hour, keep warm at 50°C, continuously take samples to check the content of the reaction solution, the conversion rate of prenaldehyde is 99.2% after 1.5 h, stop stirring.
[0055] (4) Separation of catalysts
[0056] After cooling down, the r...
Embodiment 2
[0068] Embodiment 2 A kind of method for preparing methyl heptenone by isopentenal
[0069] condensation step
[0070] (1) Preparation of solid catalyst
[0071] Using γ-alumina as a carrier, NaOH / Al with a mass percentage of NaOH of 15% was prepared by equal volume impregnation method 2 o 3 Solid base catalyst, dried in an oven at 100-120°C for 24 hours, and roasted in a muffle furnace at 500°C for 3 hours.
[0072] (2) Add acetone and catalyst
[0073] Add 150.0g of acetone to a 1000ml three-neck flask with mechanical stirring and a condenser, and add 10g of NaOH with a mass percentage of 15% NaOH / Al 2 o 3 Solid base catalyst, mechanical stirring.
[0074] (3) Drop prenaldehyde
[0075] Add 181.0 g prenaldehyde dropwise at 50°C for 1 hour, keep warm at 50°C, continuously take samples to check the content of the reaction solution, after 1.2 hours the conversion rate of prenaldehyde is 99.5%, stop stirring.
[0076] (4) Separation of catalysts
[0077] After cooli...
Embodiment 3
[0090] Embodiment 3 A kind of method that prepares methyl heptenone by isopentenal
[0091] condensation step
[0092] (1) Preparation of solid catalyst
[0093] Using γ-alumina as a carrier, NaOH / Al with a mass percentage of NaOH of 20% was prepared by equal volume impregnation method 2 o 3 Solid base catalyst, dried in an oven at 100-120°C for 24 hours, and roasted in a muffle furnace at 500°C for 3 hours.
[0094] (2) Add acetone and catalyst
[0095] Add 300.0g of acetone to a 1000ml three-neck flask with mechanical stirring and a condenser, and add 3g of NaOH with a mass percentage of 20% NaOH / Al 2 o 3 Solid base catalyst, mechanical stirring.
[0096] (3) Drop prenaldehyde
[0097] Add 100.0 g prenaldehyde dropwise at 55°C for 0.5 hours, keep warm at 55°C, continuously take samples to check the content of the reaction solution, after 2.0 h of heat preservation, the conversion rate of prenaldehyde is 99.1%, stop stirring.
[0098] (4) Separation of catalysts ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com