Method for synthesizing clethodim
A technology for clethodim and ketene, applied in the field of pesticide synthesis, can solve the problems of low overall yield of clethodim, small pH value range, difficult production and operation, etc., and achieves low production cost, good product quality, and little environmental pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
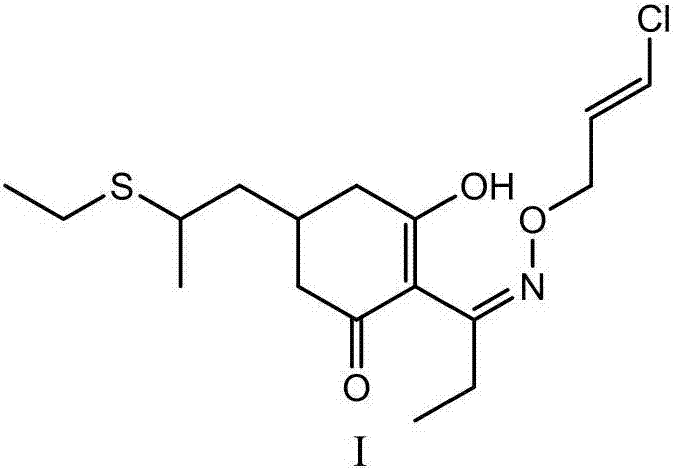
[0059] D. The preparation method of clethodim
[0060] Reaction of (±)-2-[propionyl]-3-[hydroxyl]-5-[2-(ethylthio)propyl]cyclohex-2-enone with chloroallyloxyamine to prepare clethod Ketones (Formula I):
[0061]
[0062] in:
[0063] The reaction temperature of intermediate VIII and chloroallyloxyamine is 10-50°C, more preferably 20-30°C; the reaction time is 1-5h, more preferably 2-3h. If the temperature is too high and the time is too long, impurities will increase; if the temperature is too low and the time is too short, the reaction will be incomplete.
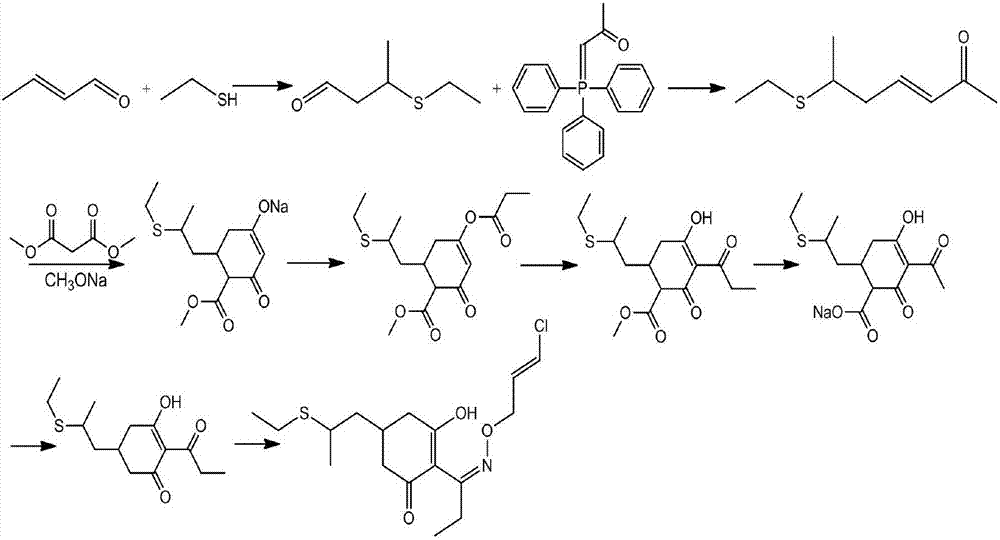
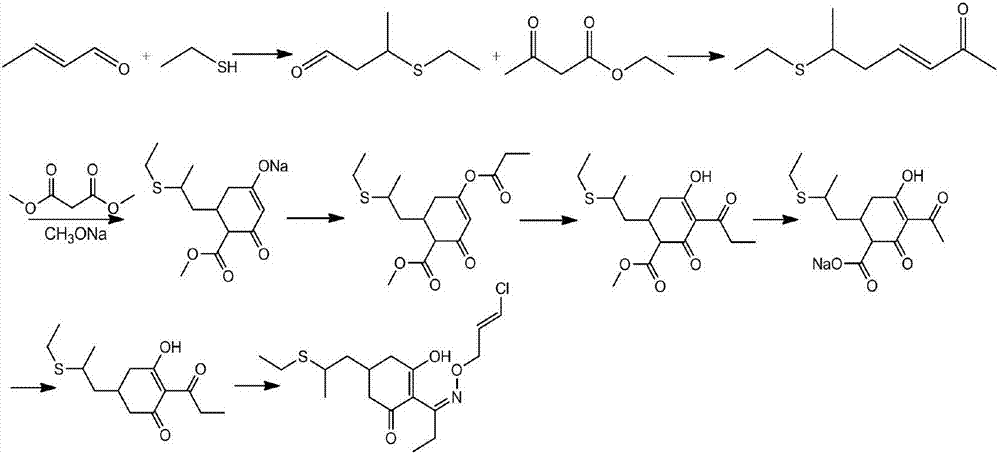
[0064] In a specific embodiment of the present invention, its reaction process is as follows:
[0065]
specific Embodiment
[0069] 1) Preparation of 6-ethylthio-3-hepten-2-one (formula III):
[0070] Add 100g (purity 97.8%, 0.888mol) of 3,5-heptadiene-2-one (formula II), 2.76g (0.027mol) triethylamine into the reaction flask, stir at room temperature (20-25°C) for 15 Minutes later, the temperature was slowly raised to 55°C, and 60.69g (0.977mol) of ethanethiol was started to be added dropwise. The temperature of the dropping process was controlled at 55-65°C. Gas chromatography traced the complete reaction (3,5-heptadien-2-one ≤ 1%), lowered to room temperature (20-25°C), added 400ml of water and 1000ml of toluene, stirred for 30 minutes, stood still for 30 minutes, and separated; Add 100ml of 0.5% dilute hydrochloric acid to the organic layer, stir and wash, and let stand for liquid separation; add 200ml of water to the organic layer and stir for 30 minutes, let stand for 30 minutes, and separate the liquids. When moisture≤0.1%, stop water separation and cool to room temperature, obtain 1011g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com