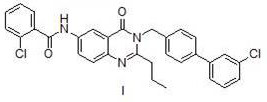
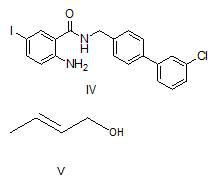
Method for preparing microsomal prostaglandin e2 synthase-1 inhibitor
A prostaglandin and solvent technology, applied in the field of preparing microsomal prostaglandin E2 synthase-1 inhibitor, can solve the problems of low atom economy, low reaction yield and the like, achieve broad development prospects, simple steps and economical high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The following examples are presented to illustrate certain embodiments of the invention and should not be construed as limiting the scope of the invention. Many modifications, variations and changes can be made to the disclosed content of the present invention simultaneously in terms of materials, methods and reaction conditions. All such modifications, changes and alterations are surely within the spirit and scope of the invention.
[0029] Examples of the invention are provided below:
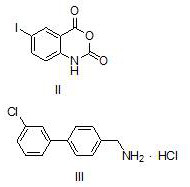
[0030] Compound III: 4-(3-chlorophenyl)benzylamine hydrochloride
[0031] 4-[(3'-chlorophenyl)phenylmethanamine hydrochloride
[0032]
[0033] Under nitrogen protection, p-bromobenzylamine (126mg, 1mmol), 3-chlorophenylboronic acid (172mg, 1.1mmol), NaF (105mg, 2mmol), potassium carbonate (311mg, 2.25mmol), ethanol 2.5ml, deoxygenated water 2.5 ml, added to a 25mL Schlenk reaction flask in turn, and reacted at room temperature for 0.5h. Then add Pd(PPh 3 ) 4 (58mg, 5mmol%) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com