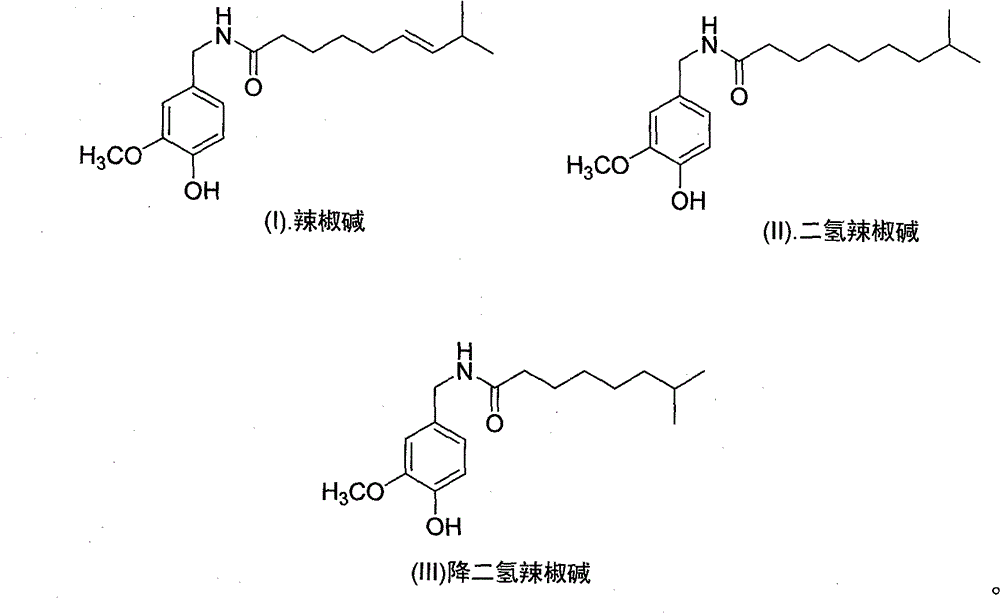
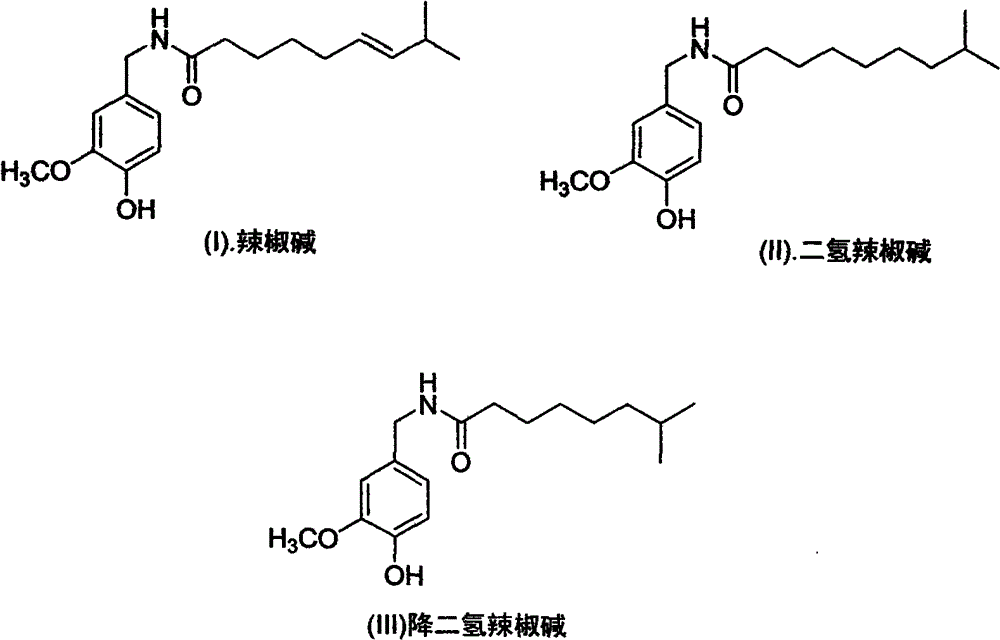
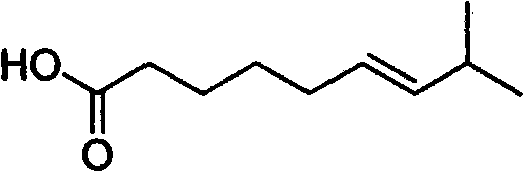
Artificial synthesis method of capsaicin homologue
A technology for artificial synthesis, capsaicin, is applied in the field of artificial synthesis of capsaicin homologues, and can solve problems such as being difficult to apply to an industrial production process, being unfriendly to the environment, and having complicated steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1. the artificial synthesis of capsaicin
[0041] (1) 4-methyl-3-hydroxy-1-pentene
[0042] A two liter round bottom flask was fitted with a reflux condenser, dropping funnel and stirrer, then 24.3 grams of magnesium bars (1.0 moles) and 1 liter of dry diethyl ether were added. Under nitrogen protection, 100 ml of 2-bromopropane (131 g, 1.06 mol) was added dropwise, and the rate of addition was controlled to maintain reflux. After the dropwise addition, heat to reflux for 20 minutes, and add dropwise a solution of 70 ml of dry acrolein in 100 ml of ether under cooling with an ice-water bath, and control the rate of addition to keep the reaction temperature below 15°C. After the dropwise addition was completed, the reaction was continued for one hour at room temperature. Slowly add 500 ml of 2N HCl under cooling in an ice-water bath, the ether solution is separated, the water phase is extracted with ether to obtain an ether extract, the ether solution and th...
Embodiment 2
[0057] The synthesis of embodiment 2. dihydrocapsaicin
[0058] (1) 6-methyl-3-hydroxy-1-heptene
[0059] A two liter round bottom flask was fitted with a reflux condenser, dropping funnel and stirrer, then 24.3 grams of magnesium bars (1.0 moles) and 1 liter of dry diethyl ether were added. 126 ml of 1-bromo-3-methylbutane (159 g, 1.05 mol) was added dropwise under nitrogen protection. Control the rate of addition to maintain reflux. After the dropwise addition, heat to reflux for 20 minutes, and add dropwise a solution of 70 ml of dry acrolein in 100 ml of ether under cooling with an ice-water bath, and control the rate of addition to keep the reaction temperature below 15°C. After the dropwise addition was completed, the reaction was continued for one hour at room temperature. Slowly add 500 ml of 2N HCl under cooling in an ice-water bath, the ether solution is separated, the water phase is extracted with ether to obtain the ether extract, the ether solution and the ethe...
Embodiment 3
[0068] Embodiment 3. the synthesis of nordihydrocapsaicin
[0069] (1) 5-methyl-3-hydroxy-1-hexene
[0070] A two liter round bottom flask was fitted with a reflux condenser, dropping funnel and stirrer, then 24.3 grams of magnesium bars (1.0 mole) and 1.0 liter of dry diethyl ether were added. 115 mL of isobutyl bromide (140 g, 1.02 mol) was added dropwise under nitrogen protection. Control the rate of addition to maintain reflux. After the dropwise addition, heat to reflux for 20 minutes, and add dropwise a solution of 70 ml of dry acrolein in 100 ml of ether under cooling with an ice-water bath, and control the rate of addition to keep the reaction temperature below 15°C. After the dropwise addition was completed, the reaction was continued for one hour at room temperature. Slowly add 500 ml of 2N HCl under cooling in an ice-water bath, the ether solution is separated, the aqueous phase is extracted with ether to obtain an ether extract, the ether solution and ether extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com