Preparation method of Avanafil intermediate
A technology of avanafil and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of low yield and large pollution, and achieve the effects of high yield, reduced production cost, and reduced pollution degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
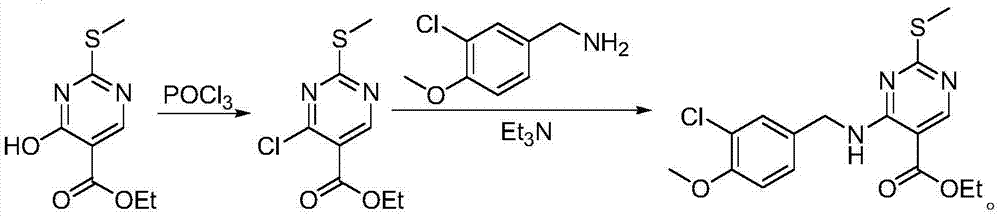
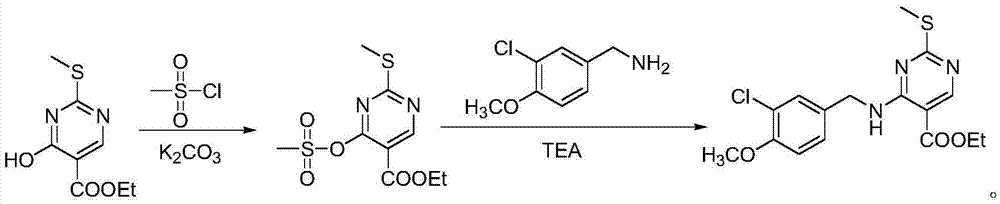
[0018] Weigh 2g of ethyl 4-hydroxy-2-methylthio-5-pyrimidinecarboxylate and add it to the reaction flask, add 10mL of DMF, place the reaction flask in a low-temperature bath at -10°C, weigh 0.94g of potassium carbonate and add it to the reaction flask bottle; weigh 1.18g of methanesulfonyl chloride, dissolve it in DMF, and slowly add the solution dropwise to the reaction flask under stirring. hours, the reaction is complete, slowly add water to the reaction solution, add ethyl acetate, extract and separate the liquid, add anhydrous sodium sulfate to the organic phase, dry, and concentrate under reduced pressure to obtain 4-methylsulfonate-2-methylthio - 2.05 g of ethyl 5-pyrimidinecarboxylate, yield: 85%, purity: 99% (HPLC, area normalization). 1 HNMR(d 6 -DMSO): δ1.41(t, J=6.0Hz, 3H), 2.62(s, 3H), 3.54(s, 3H), 4.41(q, J=6.0Hz, 2H), 9.06(s, 1H) .
[0019] Weigh 1.90g of ethyl 4-methylsulfonate-2-methylthio-5-pyrimidinecarboxylate and add it to the reaction flask, add 10mL o...
Embodiment 2
[0021] Weigh 2g of ethyl 4-hydroxy-2-methylthio-5-pyrimidinecarboxylate and add it to the reaction flask, add 10mL of DMF, place the reaction flask in a low-temperature bath at -10°C, weigh 0.94g of potassium carbonate and add it to the reaction flask bottle; weigh 1.60g of methanesulfonyl chloride, dissolve it in DMF, slowly add the solution dropwise to the reaction bottle under stirring, after the dropwise addition, raise the reaction temperature to 0°C, and stir at 0°C for 4 hours, the reaction is complete, slowly add water to the reaction solution, add ethyl acetate, extract and separate the liquid, add anhydrous sodium sulfate to the organic phase, dry, and concentrate under reduced pressure to obtain 4-methylsulfonate-2-methylthio - 2.05 g of ethyl 5-pyrimidinecarboxylate, yield: 85%, purity: 99% (HPLC, area normalization).
[0022] Weigh 1.90g of ethyl 4-methylsulfonate-2-methylthio-5-pyrimidinecarboxylate into the reaction flask, add 10mL of DMF, weigh 1.33g of (3-chlo...
Embodiment 3
[0024] Weigh 2g of ethyl 4-hydroxy-2-methylthio-5-pyrimidinecarboxylate and add it to the reaction flask, add 10mL of DMF, place the reaction flask in a low-temperature bath at -10°C, weigh 0.94g of potassium carbonate and add it to the reaction flask bottle; weigh 3.21g of methanesulfonyl chloride, dissolve it in DMF, slowly add the solution dropwise to the reaction bottle under stirring, after the dropwise addition, raise the reaction temperature to 0°C, and stir at 0°C for 4 hours, the reaction is complete, slowly add water to the reaction solution, add ethyl acetate, extract and separate the liquid, add anhydrous sodium sulfate to the organic phase, dry, and concentrate under reduced pressure to obtain 4-methylsulfonate-2-methylthio - 2.05 g of ethyl 5-pyrimidinecarboxylate, yield: 85%, purity: 99% (HPLC, area normalization).
[0025] Weigh 1.90g of ethyl 4-methylsulfonate-2-methylthio-5-pyrimidinecarboxylate into the reaction flask, add 10mL of DMF, weigh 2.65g of (3-chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com