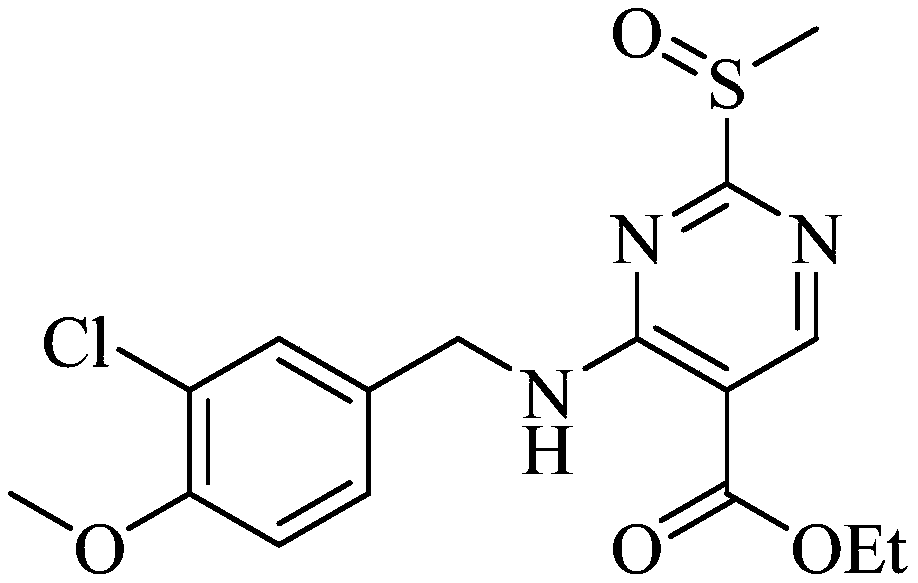
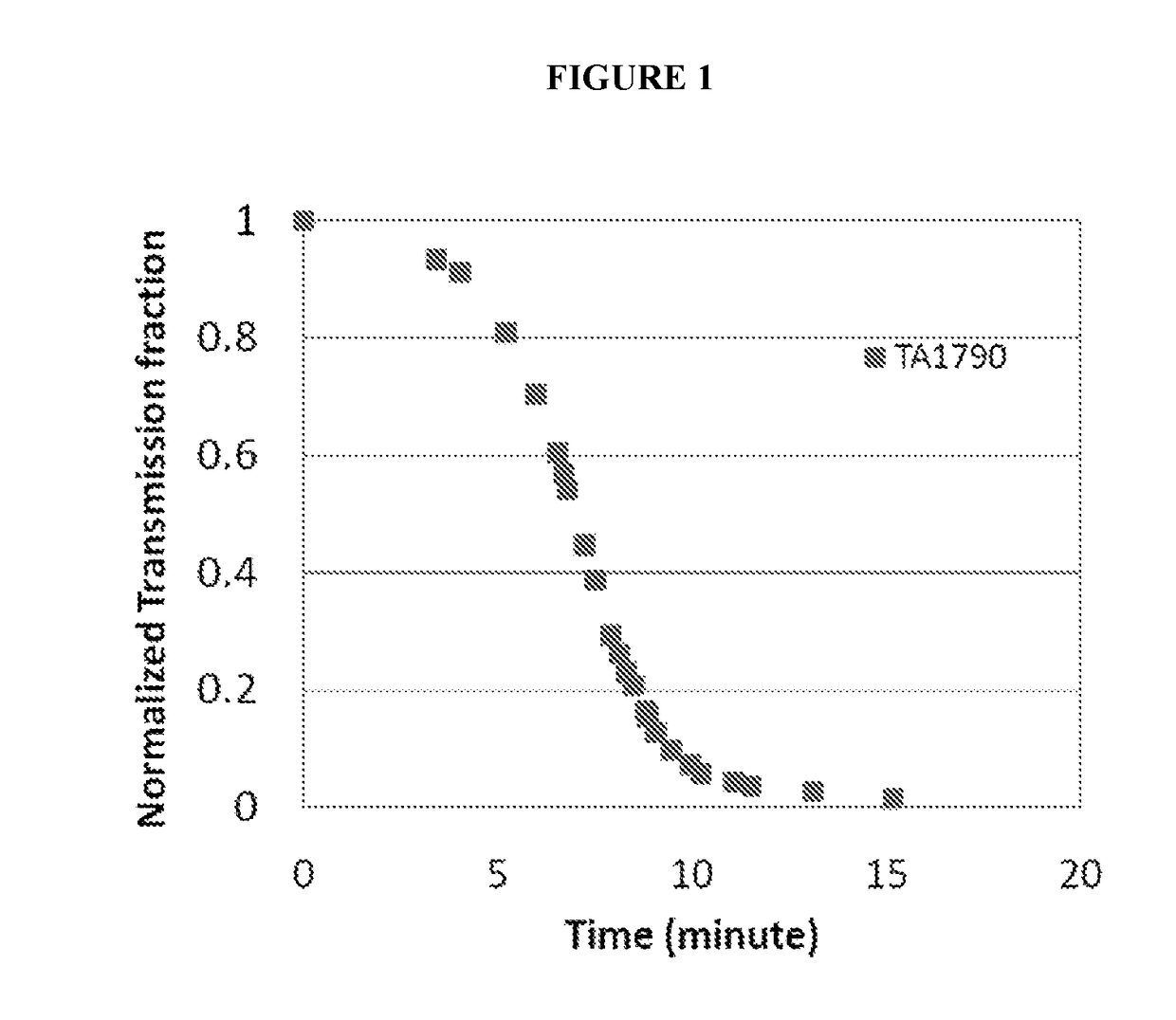
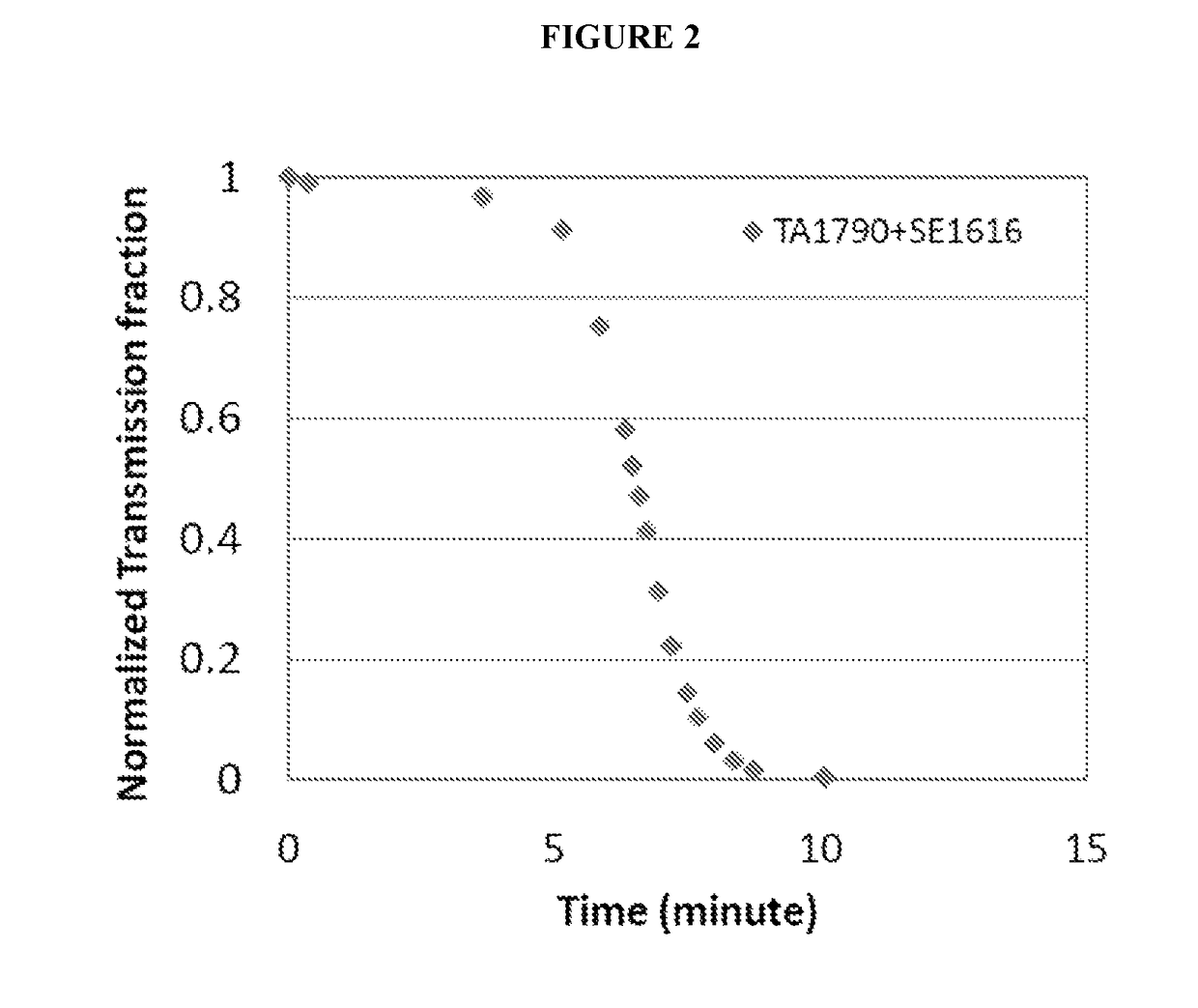
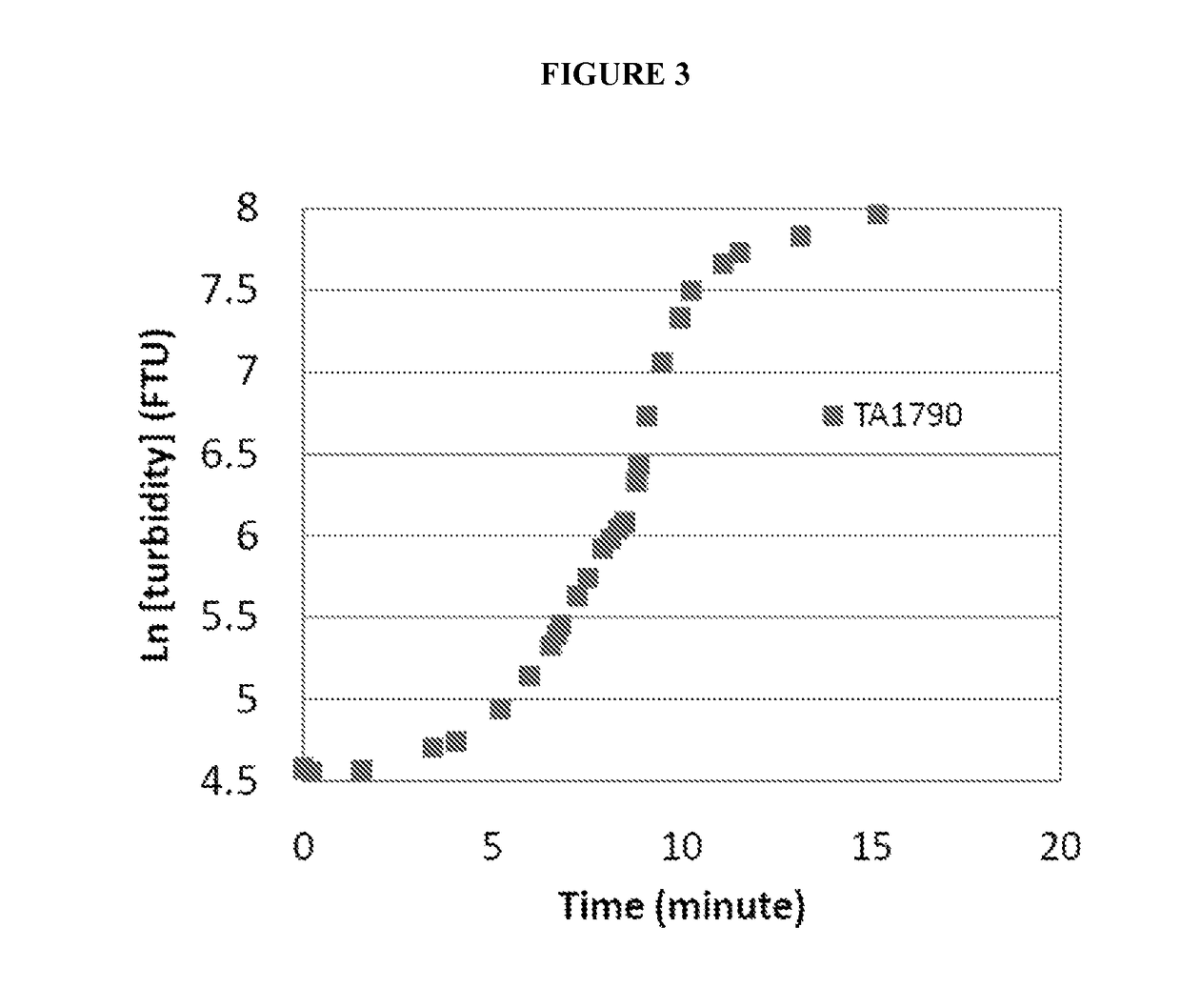
Patents
Literature
55 results about "Avanafil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
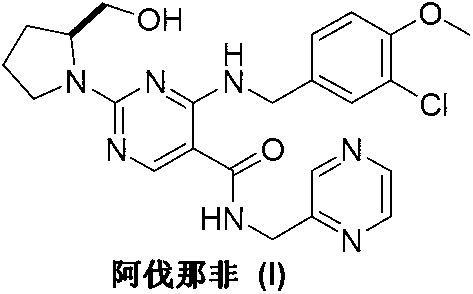
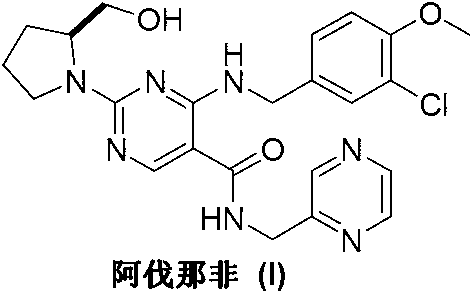
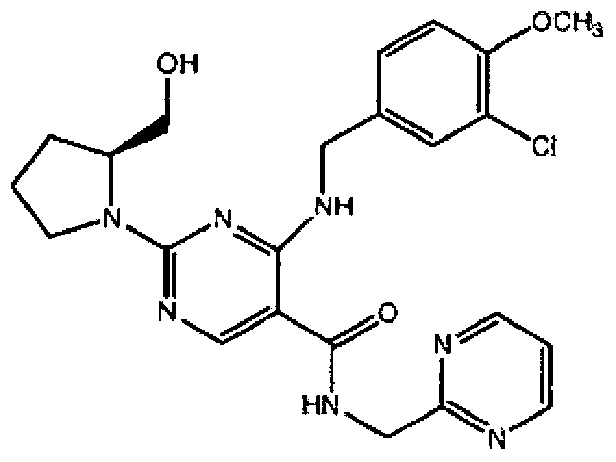
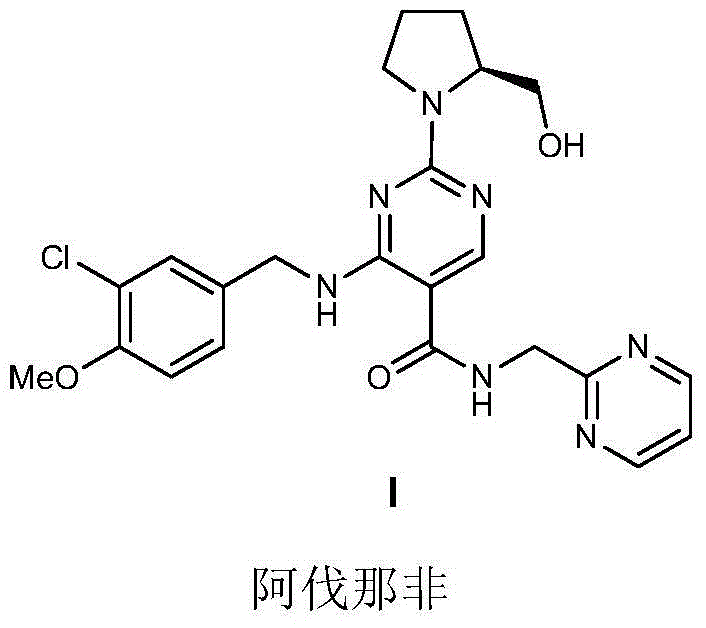
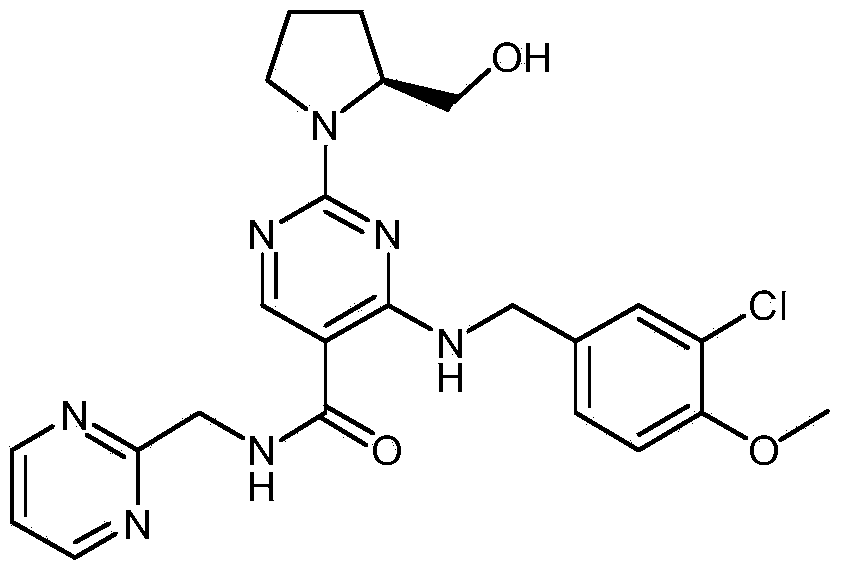
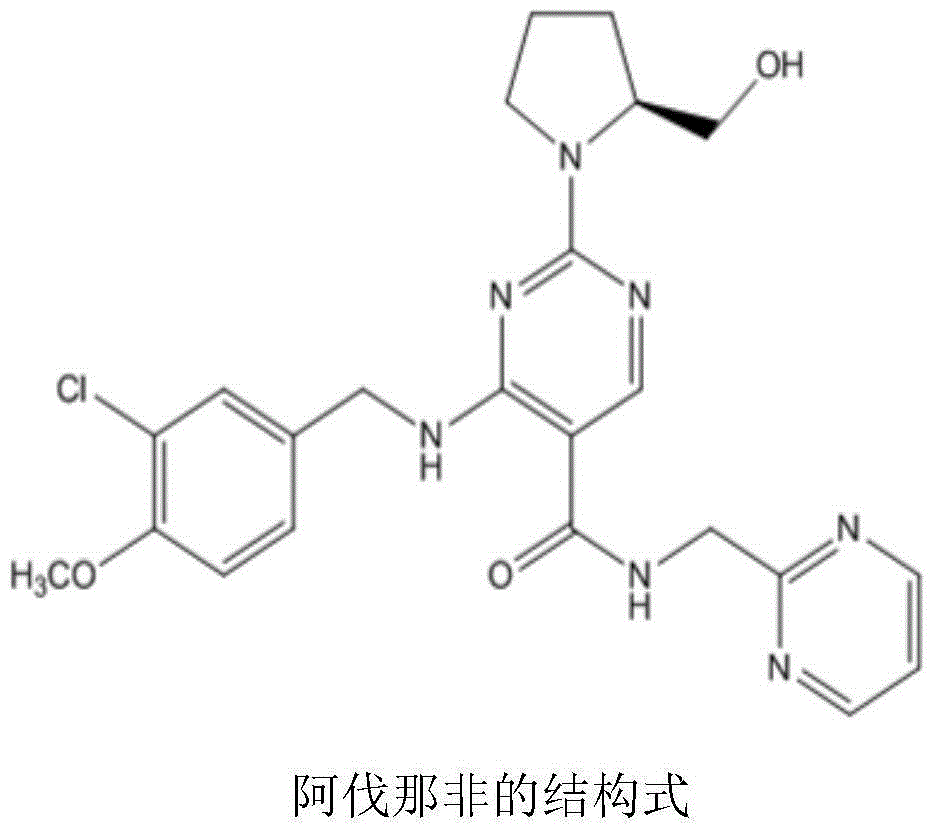
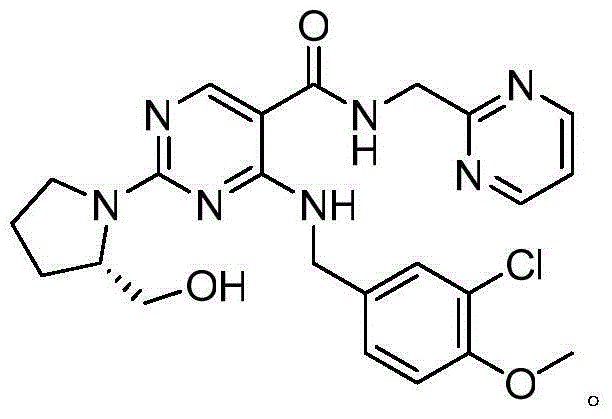
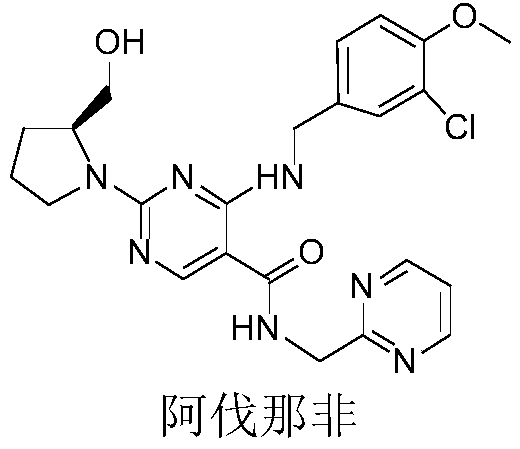
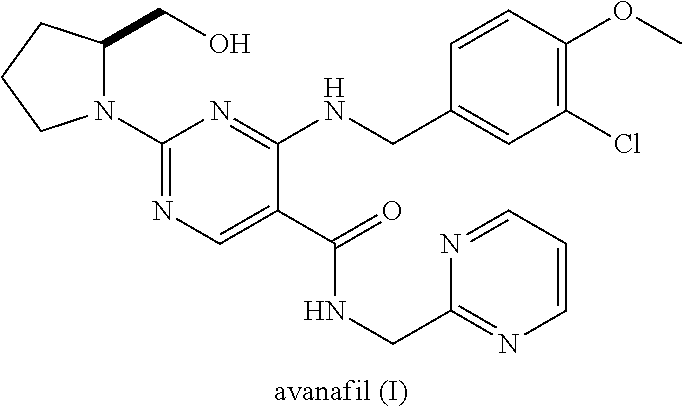
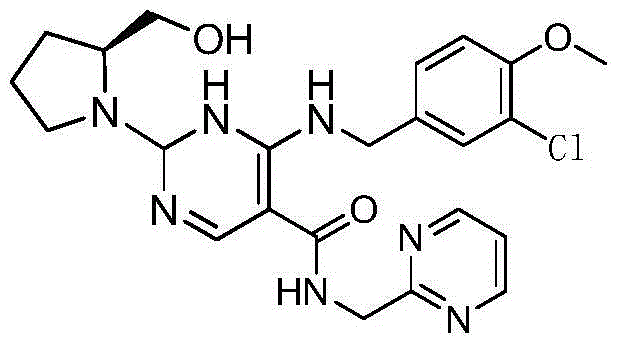
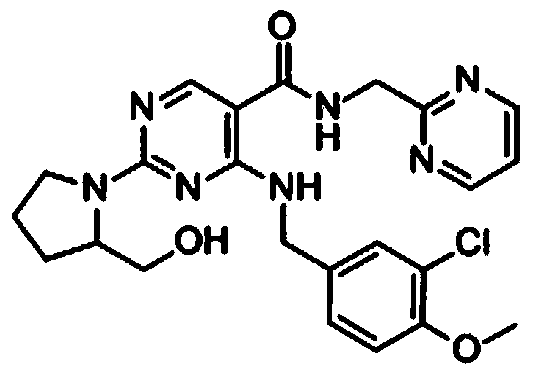
Avanafil is used to treat male sexual function problems (impotence or erectile dysfunction-ED).
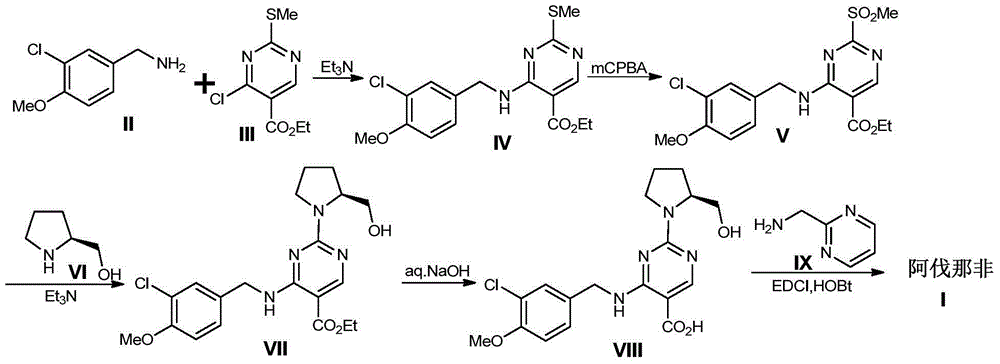
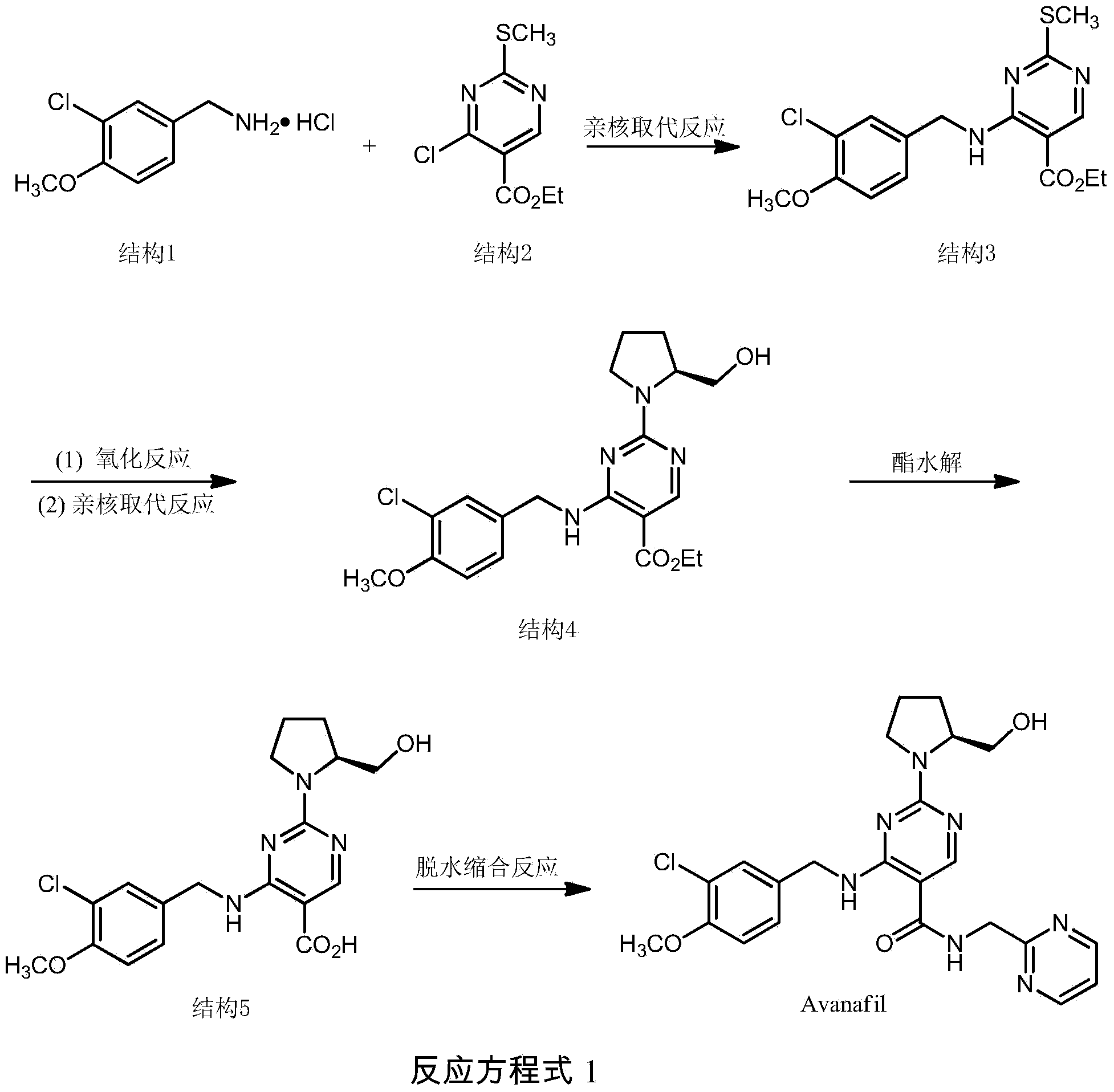
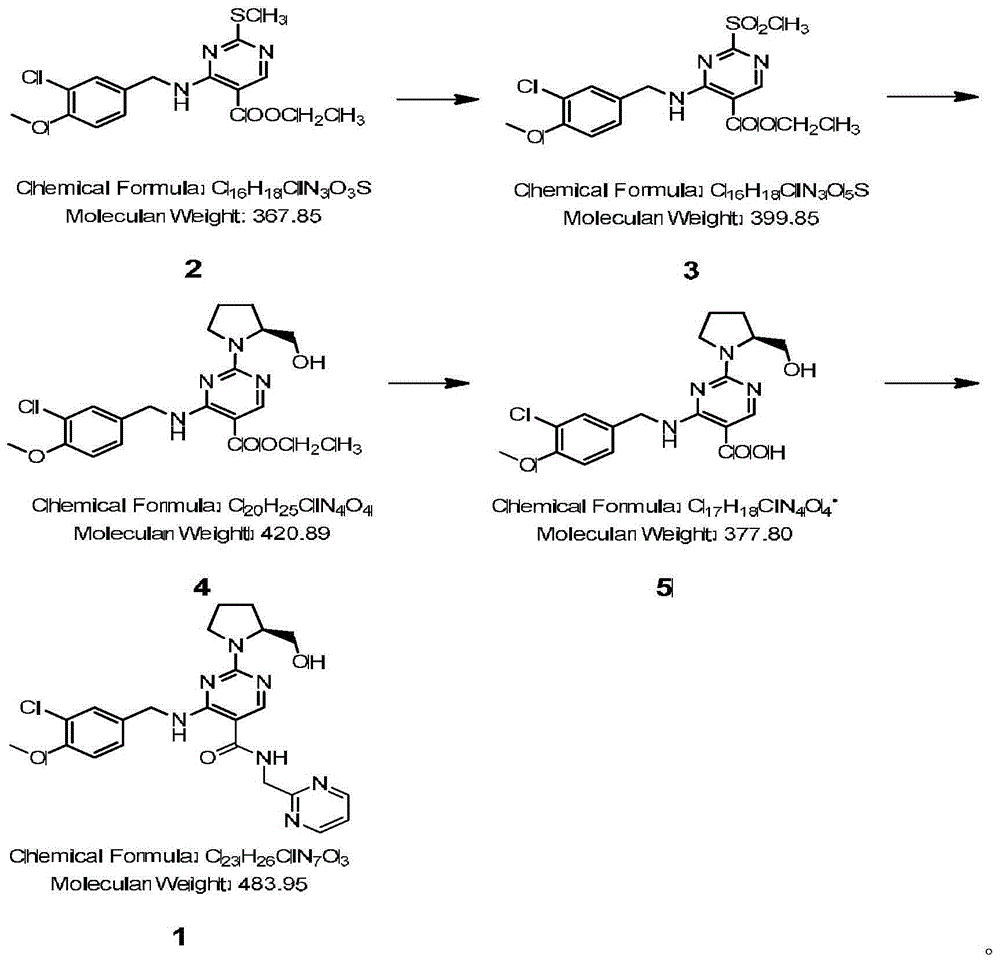
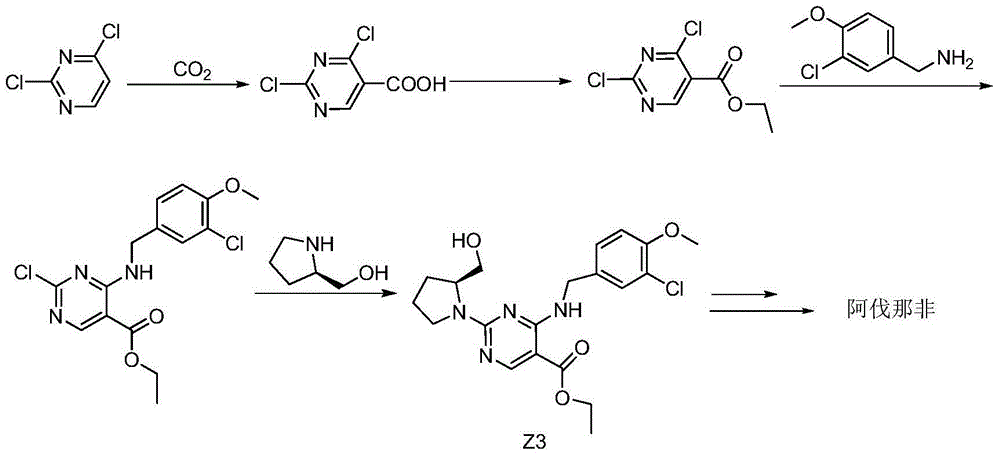
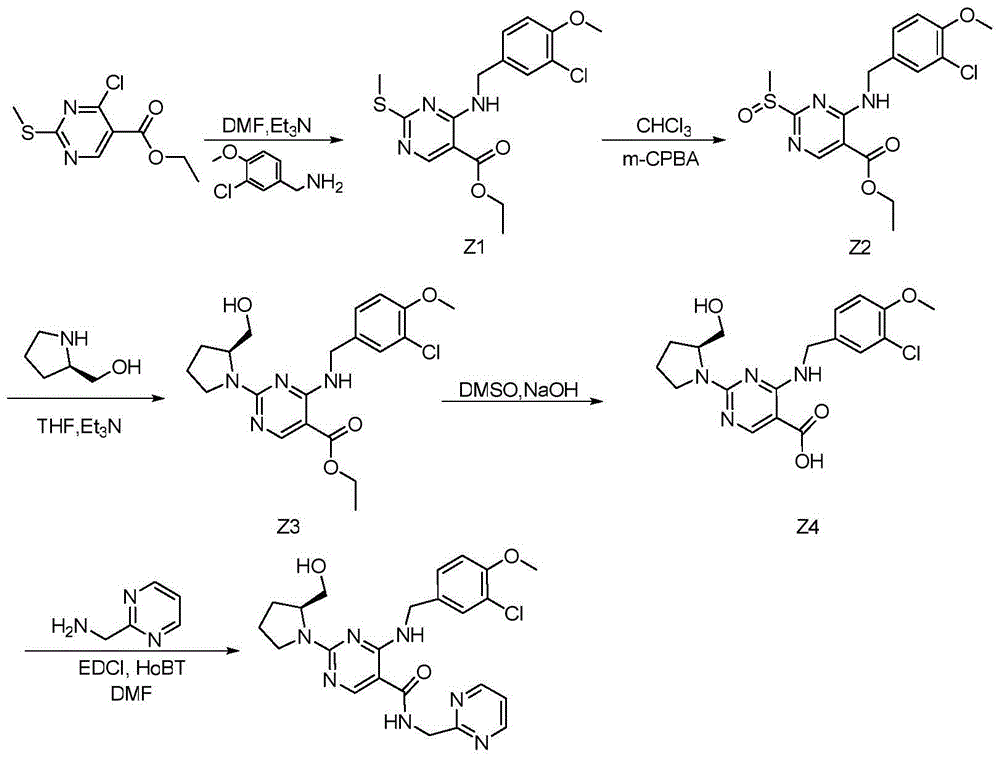
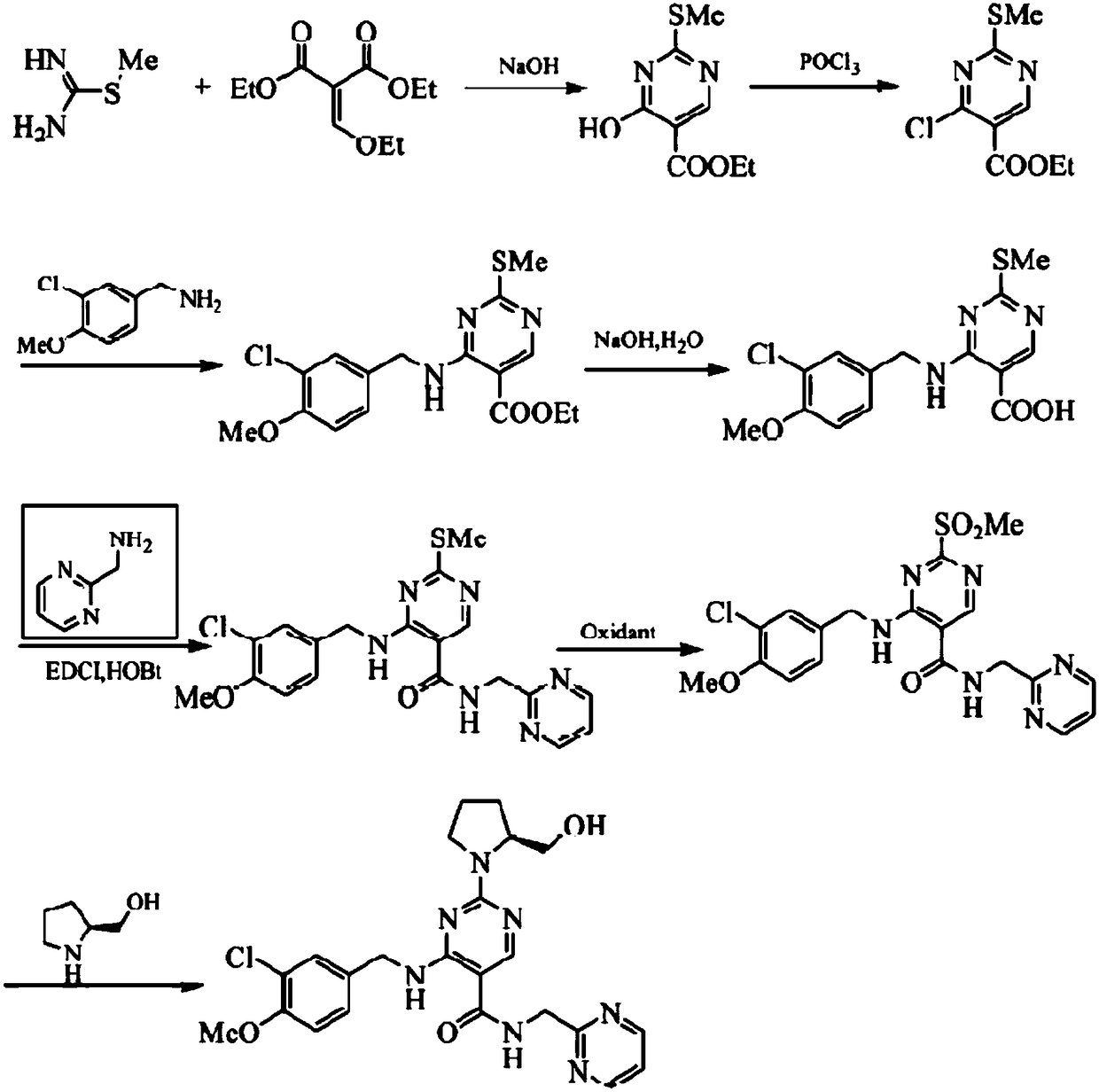
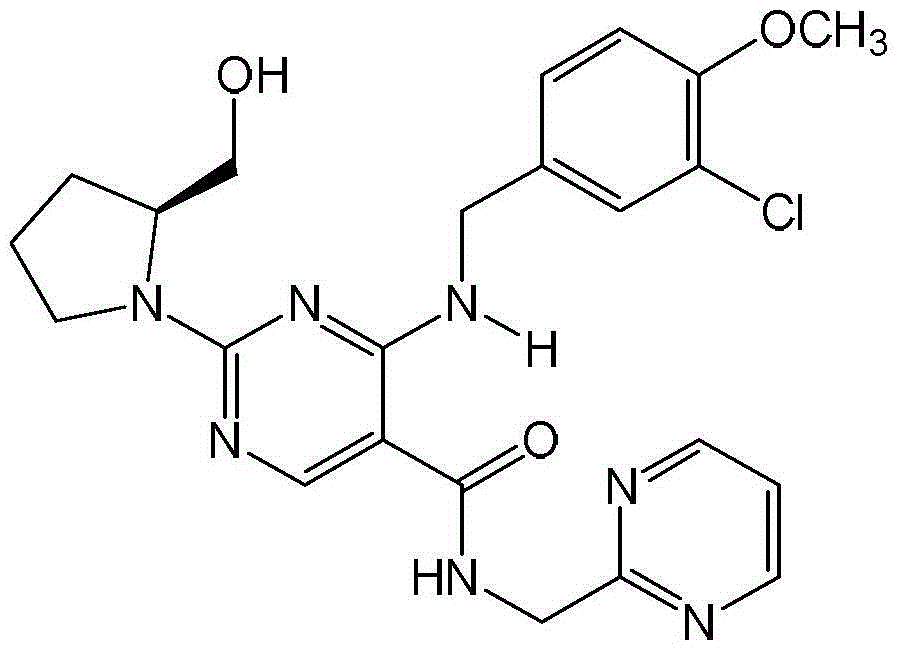
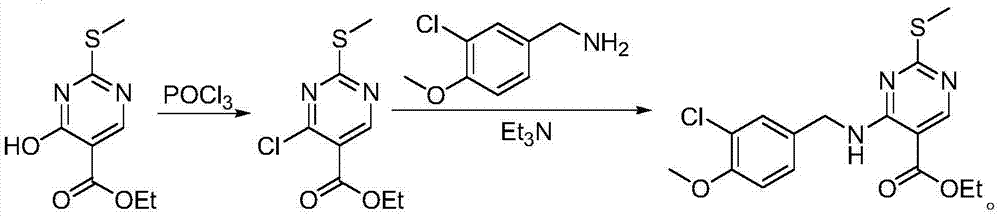
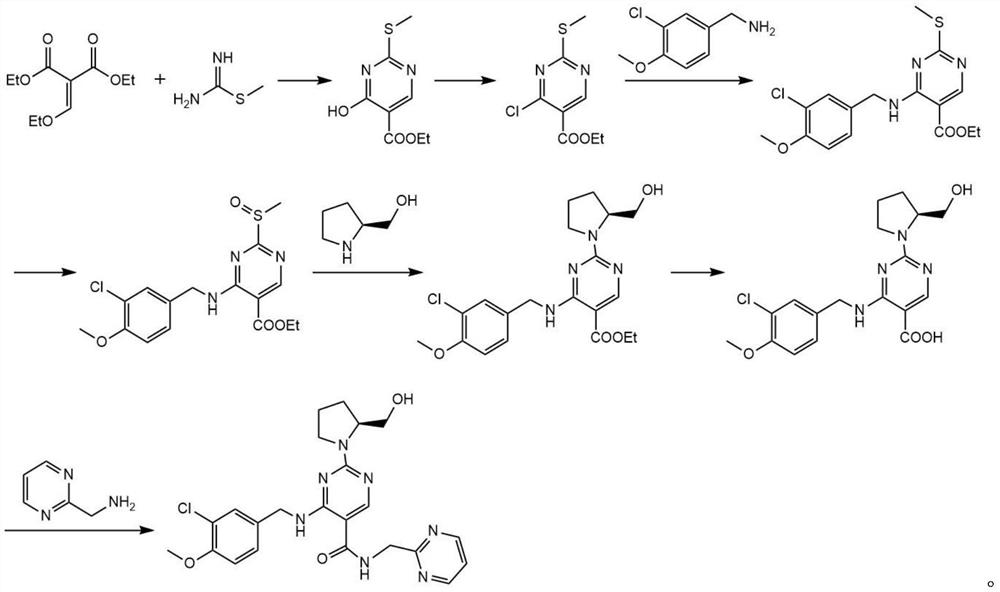
Method for preparing avanafil
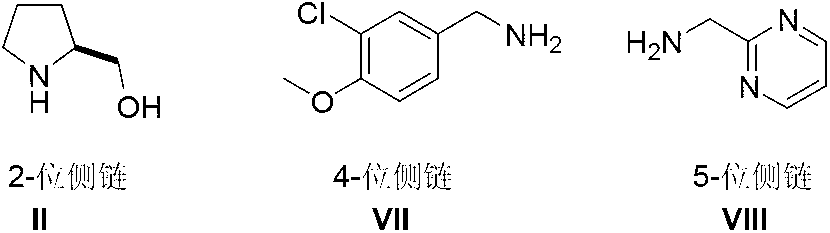
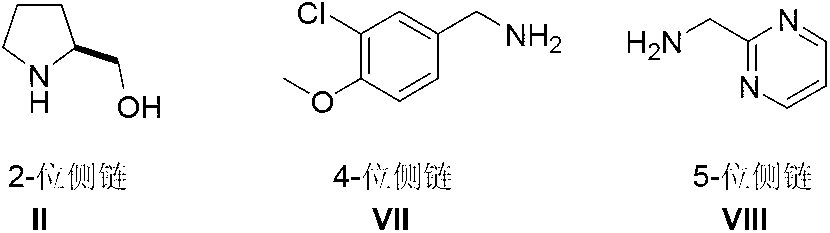
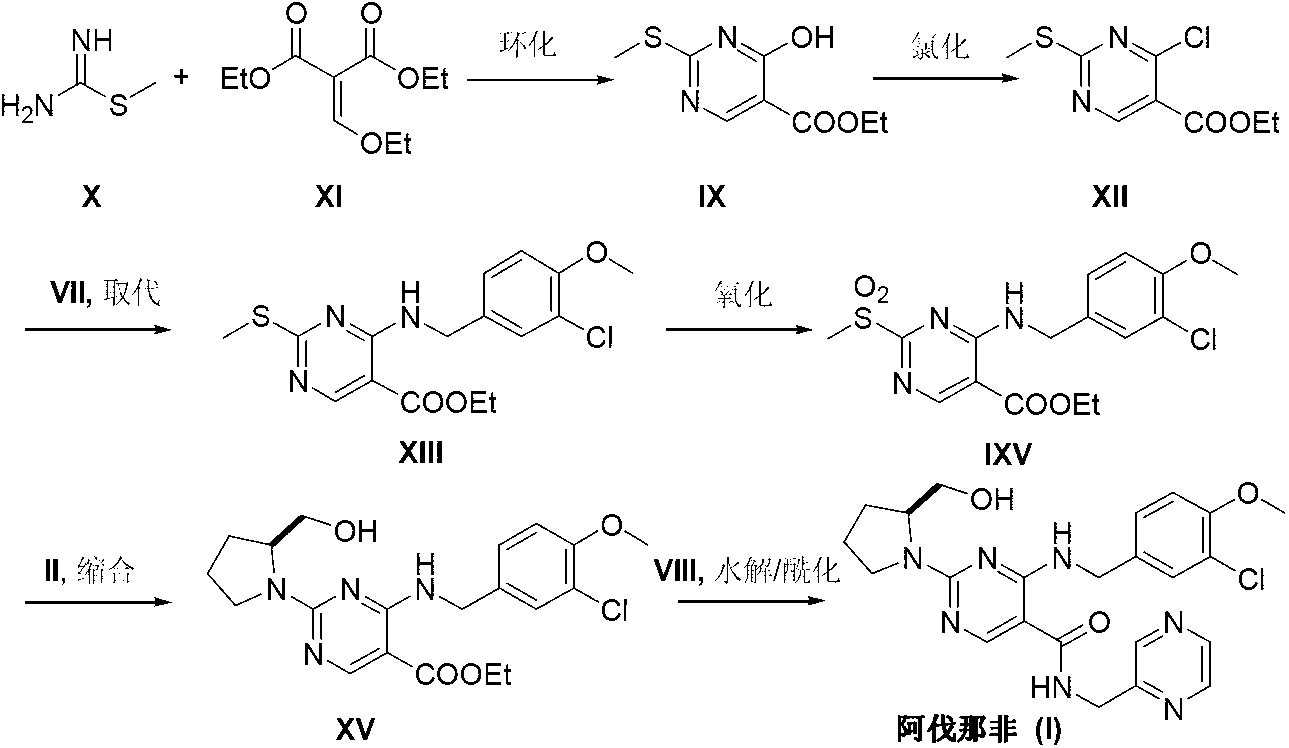
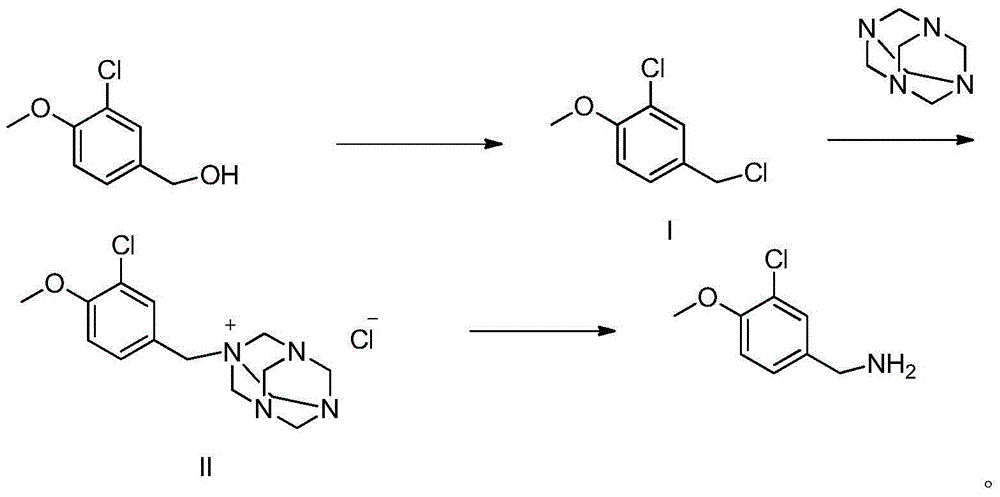
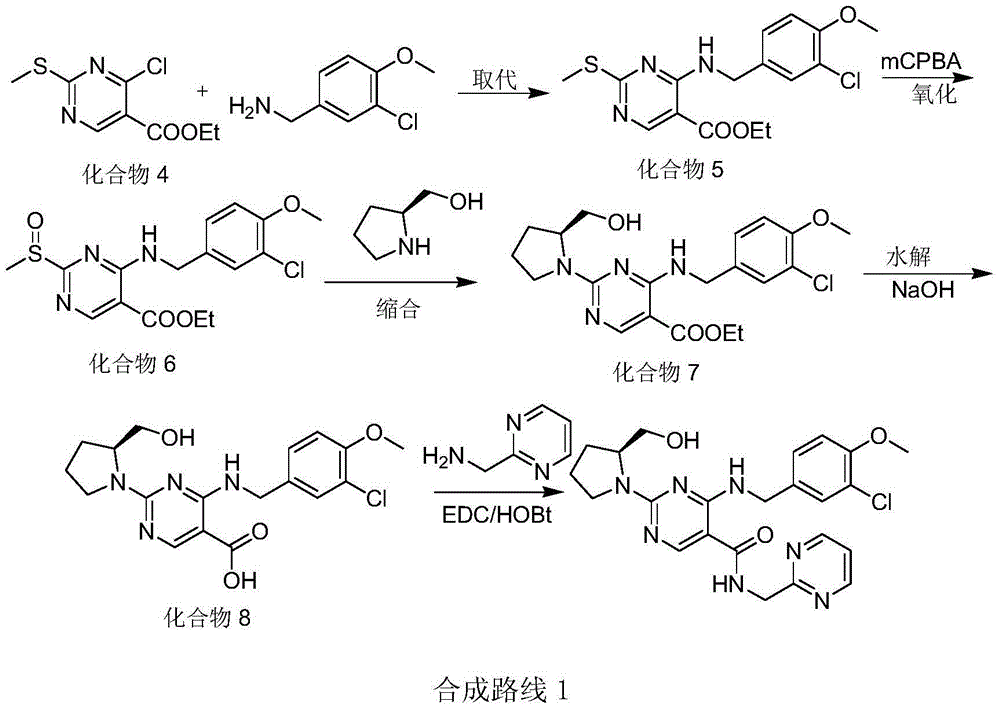
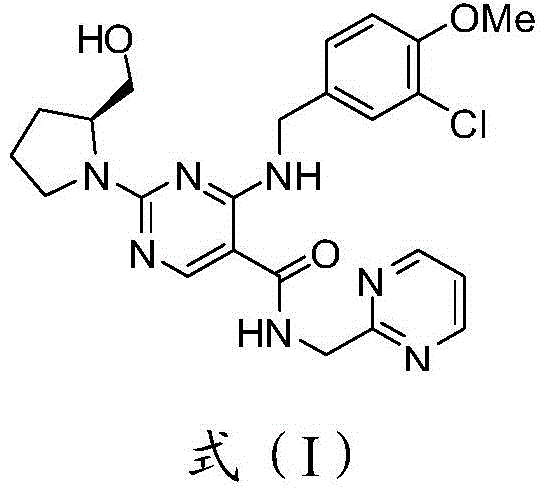
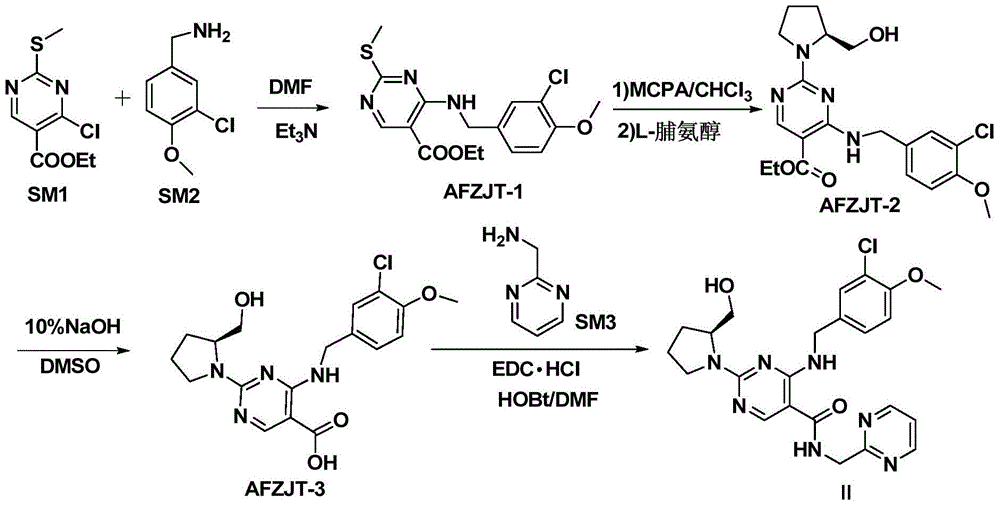
The invention discloses a method for preparing avanafil (Avanafil, I). The method comprises the steps of taking cytosine as an initial material; and orderly carrying out replacement, halogen addition and condensation reaction on a side chain 3-chlorine-4-methoxy benzyl halide (III), N-(2-methylpyrimidine) formamide (IV) and S-hydroxymethyl pyrrolidine (II), so as to obtain a target product avanafil (I). The preparation method is available in material, concise in technology, economic and environment-friendly, and suitable for the demands of industrial amplification.
Owner:江苏兰亭市政园林有限公司
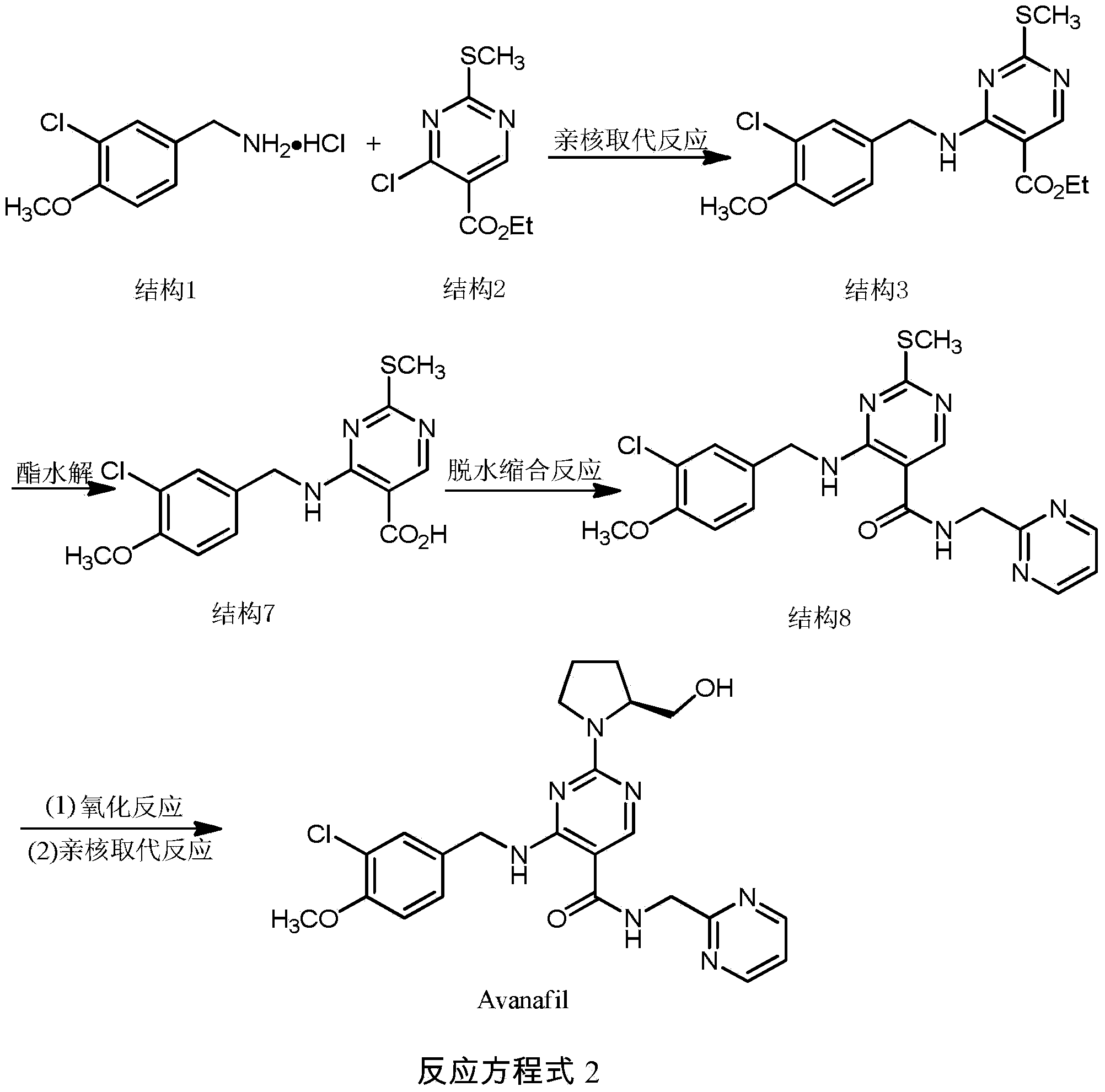
Preparation method of Avanafil
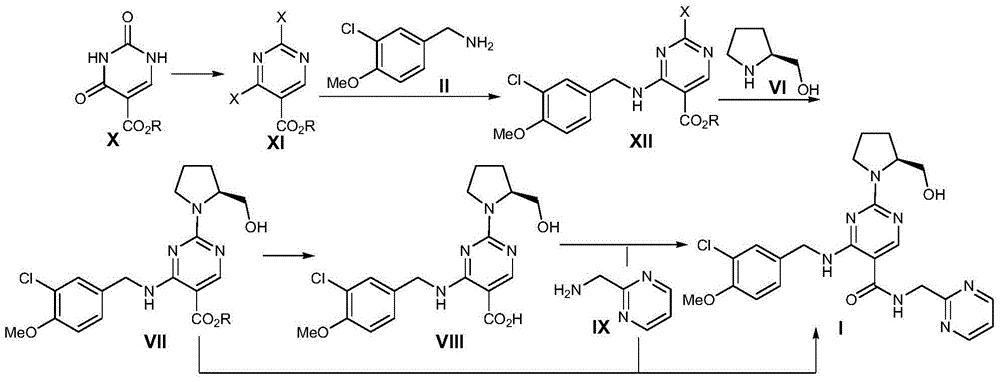
The invention discloses a preparation method of Avanafil (I). Cytosine is taken as a starting material, and substitution, condensation and halogenated addition reactions are sequentially carried out by virtue of 3-chloro-4-methoxybenzyl halogen (III), S-hydroxymethyl pyrrolidine (II) and N-(2-methyl pyrimidine) formamide (IV), thus the target product Avanafil (I) can be obtained. The preparation method has the advantages of available raw materials, simple process, economy and environmental protection and is applicable to the requirement of industrialized production.
Owner:迁安华韵知识产权服务中心
Novel intermediate used for preparation of avanafil and preparation method thereof
ActiveCN104059025AAvoid racemizationReduce usageOrganic chemistryChemical reactionBiochemical engineering
The invention provides a novel intermediate IV which is used for preparation of avanafil. The intermediate has a general formula IV as described in the specification; and in the general formula IV, R represents a C1-20 alkylsulfinyl group or a C1-20 alkylsulfonyl group. The intermediate has high purity, is suitable for industrial production and can be subjected to a one-step chemical reaction to prepare avanafil. The invention also provides a preparation method for the intermediate and a method for preparing avanafil from the intermediate.
Owner:REGENEX PHARMA LTD
Avanafil
InactiveCN103483323ASimple structureImprove product qualityOrganic chemistryRoom temperature4-methoxybenzylamine
The invention provides an avanafil intermediate A and an avanafil intermediate B, and a synthetic method of the avanafil intermediates A and B and avanafil. The synthetic method of the avanafil comprises the following steps: reacting a compound with the formula (I) with 2-methylaminopyrimidine at a temperature ranging from -10 DEG C to 5 DEG C to obtain the avanafil intermediate A; agitating the avanafil intermediate A with 3-chloro-4-methoxybenzylamine at the temperature ranging from 0 DEG C to 3 DEG C and reacting for 0.2-0.4 hour to obtain the avanafil intermediate B; and agitating the avanafil intermediate B with L-prolinol at the room temperature and reacting for 18-22 hours to obtain the avanafil. The structural formulas of the avanafil intermediates A and B and the compound with the formula (I) are shown in the specification. The avanafil intermediates A and B provided by the invention are simple in structure and good in product quality; the cost of the synthetic method is low; the synthetic route of the whole process is short and reaction steps are few, so that the reaction time is shortened and the yield and the purity of the avanafil intermediates A and B, and the avanafil are also improved.
Owner:SUZHOU UUGENE BIOPHARMA
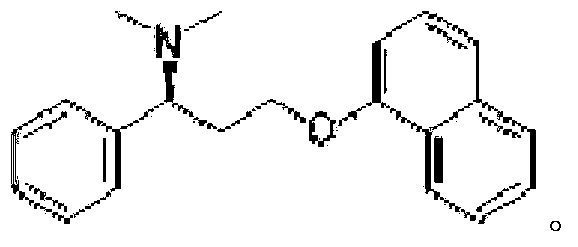
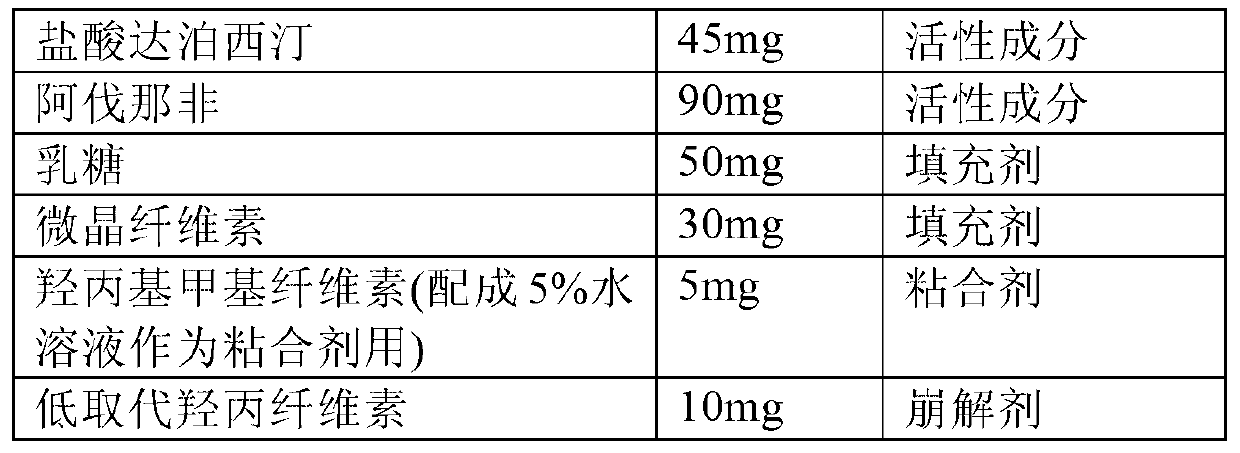
Composition for impotence and premature ejaculation
ActiveCN103340869AAvoid adverse changesAvoid adverse reactionsOrganic active ingredientsSexual disorderMedicineAvanafil
The invention relates to a composition for impotence and premature ejaculation. Specifically, the invention relates to a use of combination of dapoxetine or medical salts thereof and avanafil in preparation of a medicament for preventing or treating impotence and premature ejaculation, and further relates to a composition including the dapoxetine or medical salts thereof and avanafil. The composition disclosed by the invention has the characteristic of being excellent.
Owner:北京元延医药科技股份有限公司
Preparation method of avanafil
ActiveCN104650045AQuality improvementThe reaction is easy to operateOrganic chemistryUracilCarboxylic acid
The invention relates to a preparation method of avanafil and a new compound provided in a preparation process. According to the method, 5-uracil carboxylic acid or an ester thereof is taken as the raw material, and the avanafil meeting the clinical requirements can be synthesized at a relatively cost; besides, the preparation method is simple and convenient to operate, mild in reaction conditions, high in yield, low in cost, environmentally friendly and suitable for industrial large-scale production of the avanafil.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
Method for preparing avanafil
InactiveCN103833736AHigh melting pointImprove physical and chemical propertiesOrganic chemistryBiochemical engineeringCombinatorial chemistry
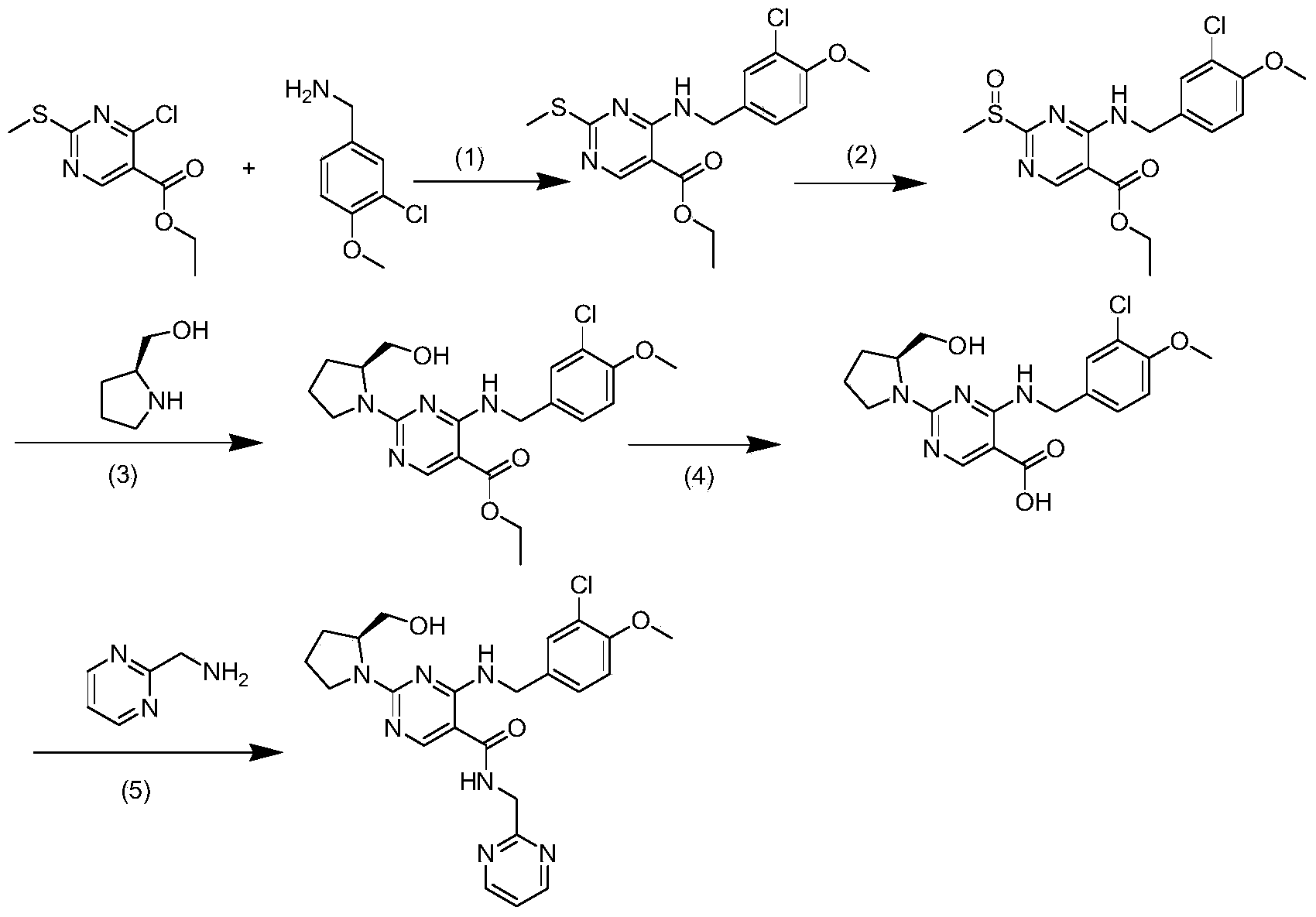
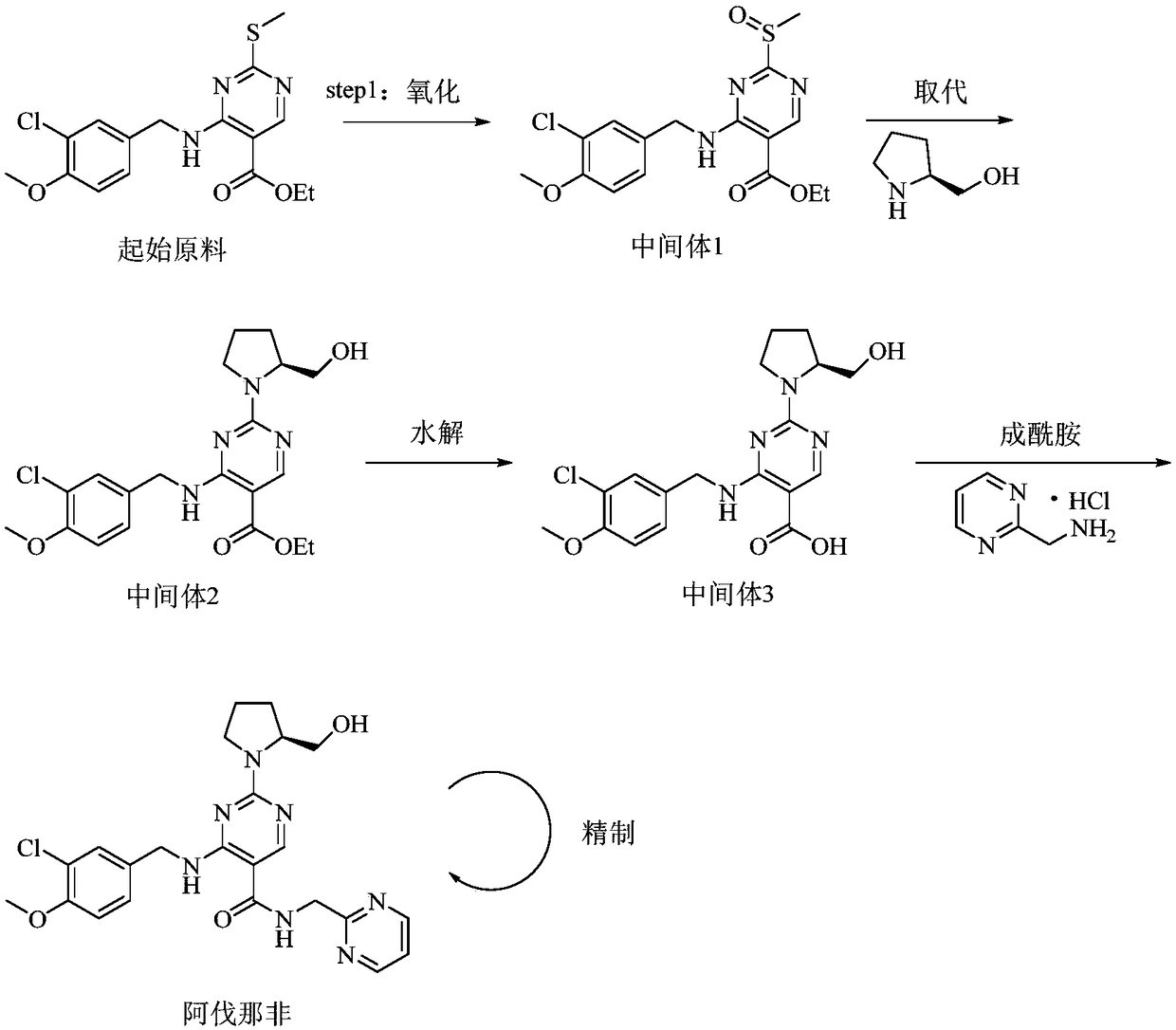
The invention relates to a method for preparing avanafil. The method is characterized by comprising the step of enabling 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine to be subjected to hydrolysis, condensation, oxidation and substitution reaction sequentially, thereby preparing avanafil. The method has the advantages of being simple and convenient in operation, easy in product quality control, high in reaction yield, high in product purity, low in cost and applicable to industrial production and the like.
Owner:BEIJING AOHE DRUG RES INST +2
Synthesis method of avanafil
InactiveCN104003981AHigh purityRaw materials are cheap and easy to getOrganic chemistryChemical synthesisSynthesis methods
The invention relates to the field of the organic chemistry synthesis and in particular relates to a synthesis method of avanafil. The method comprises the following steps: carrying out nucleophilic substitution on raw materials which are 4-chloro-5-ethoxycarbonyl-2-methylthiopyrimidine and 3-chloro-p-methoxy benzyl amine hydrochloride to obtain 4-(3-chloro-4-methoxy benzyl amine hydrochloride)-5-ethoxycarbonyl-2-methylthiopyrimidine, then sequentially carrying out oxidation reaction, nucleophilic reaction with L-prolinol, esterolysis and dehydration synthesis to finally obtain avanafil. The method has the advantages that the used raw materials are cheap and easily available; the cost is low; the operation is simple and convenient; the prepared final product has few impurities and is of a solid state. The final product can be directly re-crystallized to obtain a pure product; the product is high in purity and suitable for industrial production.
Owner:HEBEI KANGTAI PHARMA
Avanafil preparation method
InactiveCN104530017ASolve purificationSolve the problem of racemization of chiral centersOrganic chemistryCarboxylic acidFormamide
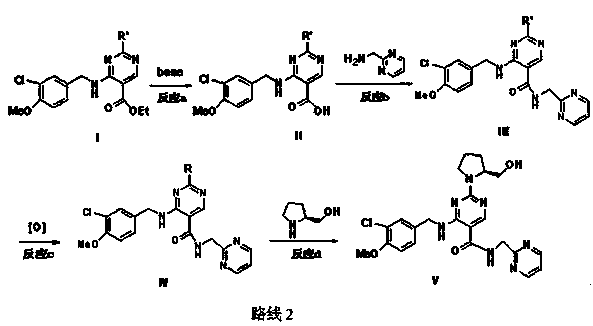
The invention relates to an avanafil preparation method. The method comprises the steps that a hydrolysis reaction is carried out on 4-(3-chlorin-4-methoxy benzyl amino)-2-methylehio pyrimidine-5-carboxylic acid ethyl ester, namely a compound (2) to obtain 4-(3-chlorin-4-methylehio pyrimidine)-2-methylehio pyrimidine-5-carboxylic acid, namely a compound (6); an acylation reaction is carried out on the compound (6) and thionyl chloride, and then the product and 2-sulfadoxine-pyrimethamine or a salt of 2-sulfadoxine-pyrimethamine are condensed to obtain 4-(3-chlorin-4-methylehio pyrimidine)-2-methylehio pyrimidine-N-(2- pyrimidine methyl)-5-pyrimidine formamide, namely a compound (7); the compound (7) is oxidized by metachloroperbenzoic acid to obtain 4-(3-chlorin-4-methylehio pyrimidine)-2-methylsulfonyl pyrimidine-N-(2-pyrimidine methyl)-5-pyrimidine formamide, namely a compound (8), a nucleophilic substitution reaction is carried out on the compound (8) and L-prolinol to obtain avanafil. The method is low in cost, easy and convenient to operate, high in product purity, high in reaction yield and suitable for industrialization scale production.
Owner:NANJING XIAOZHUANG UNIV
Avanafil intermediate as well as preparation method and application thereof
The invention discloses an avanafil intermediate as well as a preparation method and application thereof. The avanafil intermediate is a compound having a general formula as shown in the description, wherein R in the general formula is selected from C1-C4 alkyl. The preparation method of the intermediate comprises the steps a-d in the synthesis route as shown in the description. The invention also discloses an application of the intermediate. Each reaction step for preparing avanafil from the intermediate has the advantages of simple operation, mild reaction conditions, easily available reaction raw materials, high reaction yield and the like, the products are easy to separate and purify; the total yield of prepared avanafil is increased to 40% and the HPLC purity reaches up to 99.8%; the preparation cost of avanafil is greatly reduced, the quality of avanafil is ensured, and thus the intermediate is very much in line with industrial production requirements and has practical value.
Owner:重庆瑞泊莱医药科技有限公司
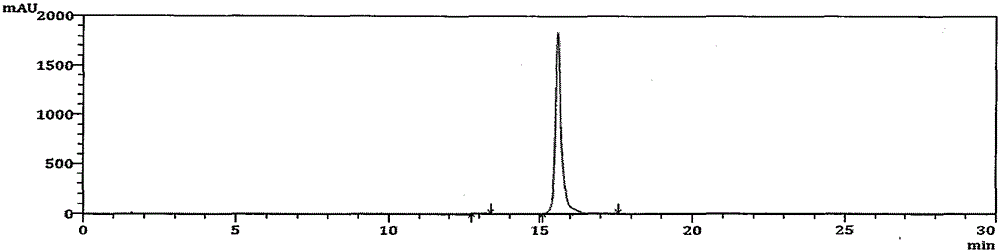
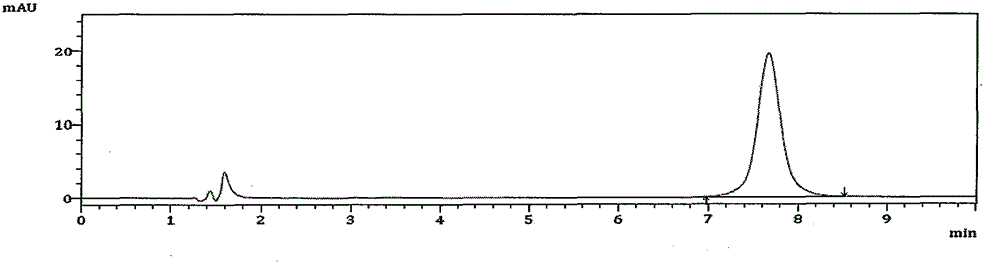
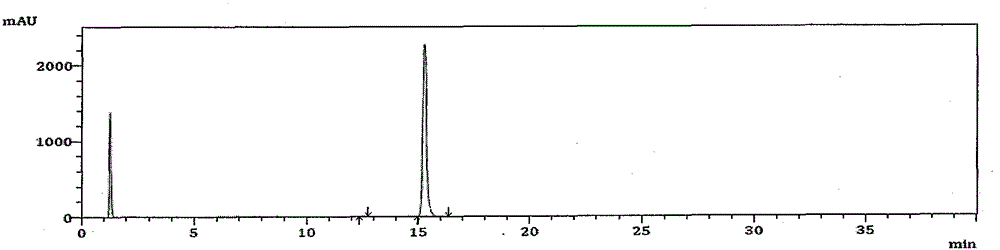
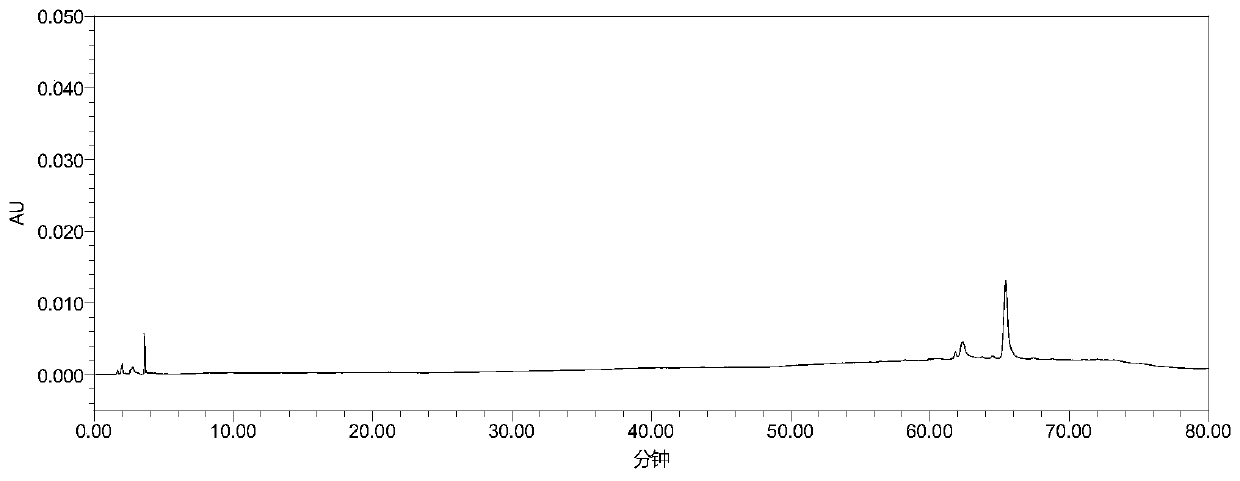
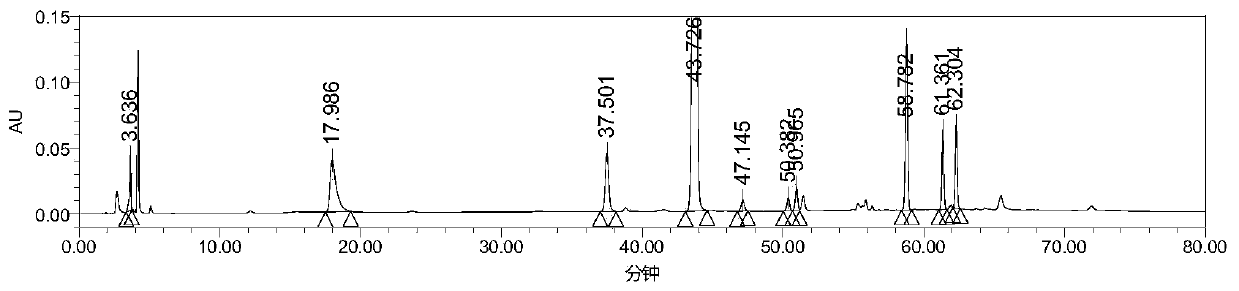
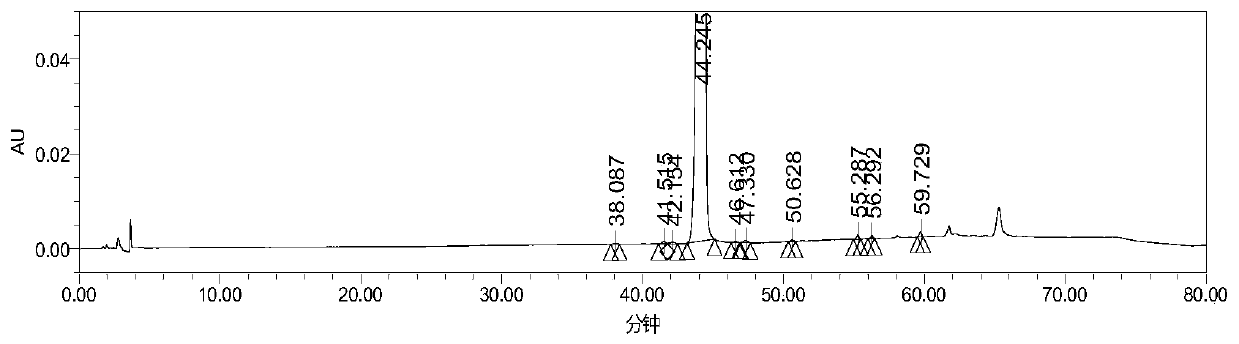
High performance liquid chromatography analysis method for avanafil and preparation thereof
InactiveCN106198766AThe analytical method is practical and reliableImprove stabilityComponent separationInjection volumeUltraviolet detectors
The present invention provides a high performance liquid chromatography analysis method for avanafil and a preparation thereof, and belongs to the technical field of drug analysis. According to the high performance liquid chromatography analysis method, a chromatographic column selecting an octadecyl-bonded silica gel as a filler is selected, the specification is 4.6 mm*150 mm, 5 [mu]m, 4.6 mm*200 mm, 5 [mu]m, 4.6 mm*250 mm, 5 [mu]m, 4.6 mm*150 mm, 3.5 [mu]m, 4.6 mm*200 mm, 3.5 [mu]m, 4.6 mm*250 mm, 3.5 [mu]m, the mobile phase is an acetonitrile-buffer salt according to a ratio of 30-70:70-30, the flow rate is 5-1.5 ml / min, the column temperature is 30-40 DEG C, the wavelength of an ultraviolet detector is 200-300 nm, and the injection volume is 5-50 [mu]l. According to the present invention, the method only uses the ordinary liquid chromatograph, the requirement on the equipment is not high, the two mediums selected by the mobile phase are generally easy to obtain, the feasibility is high, the operation process is simple and convenient, the applicability is good, and the method can be widely used in avanafil and the preparation thereof.
Owner:南京从一医药科技有限公司
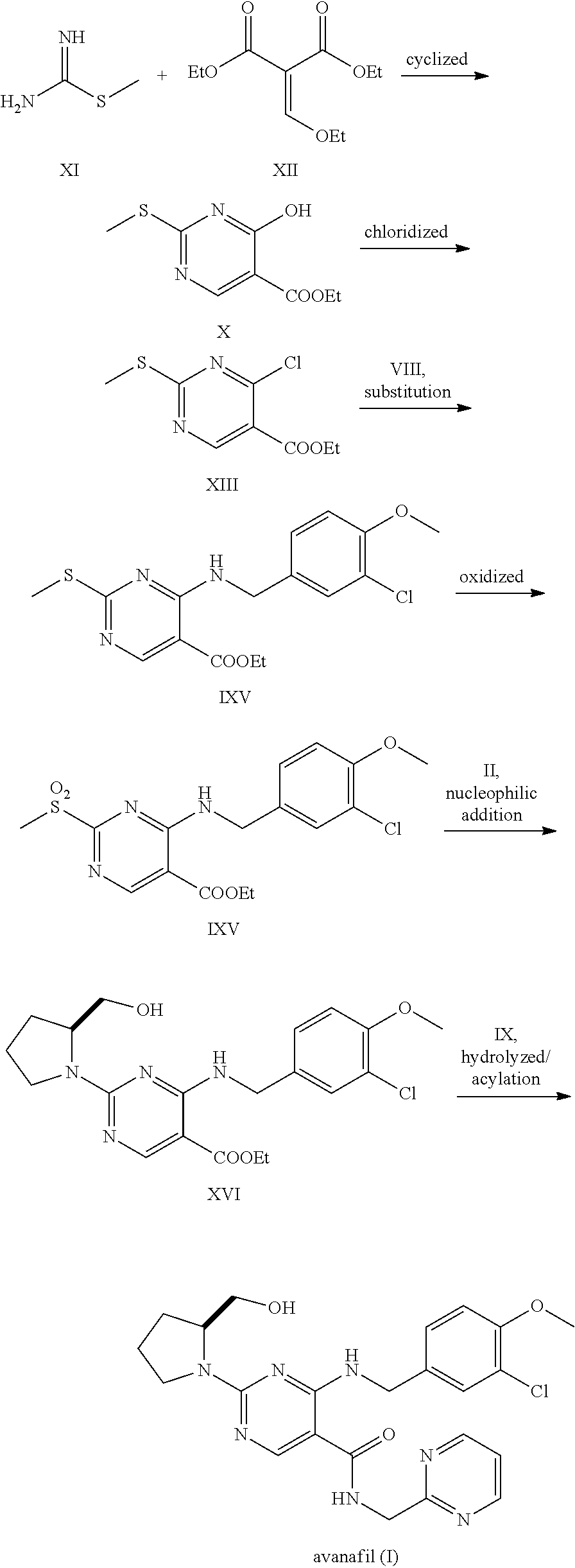
Preparation method of avanafil
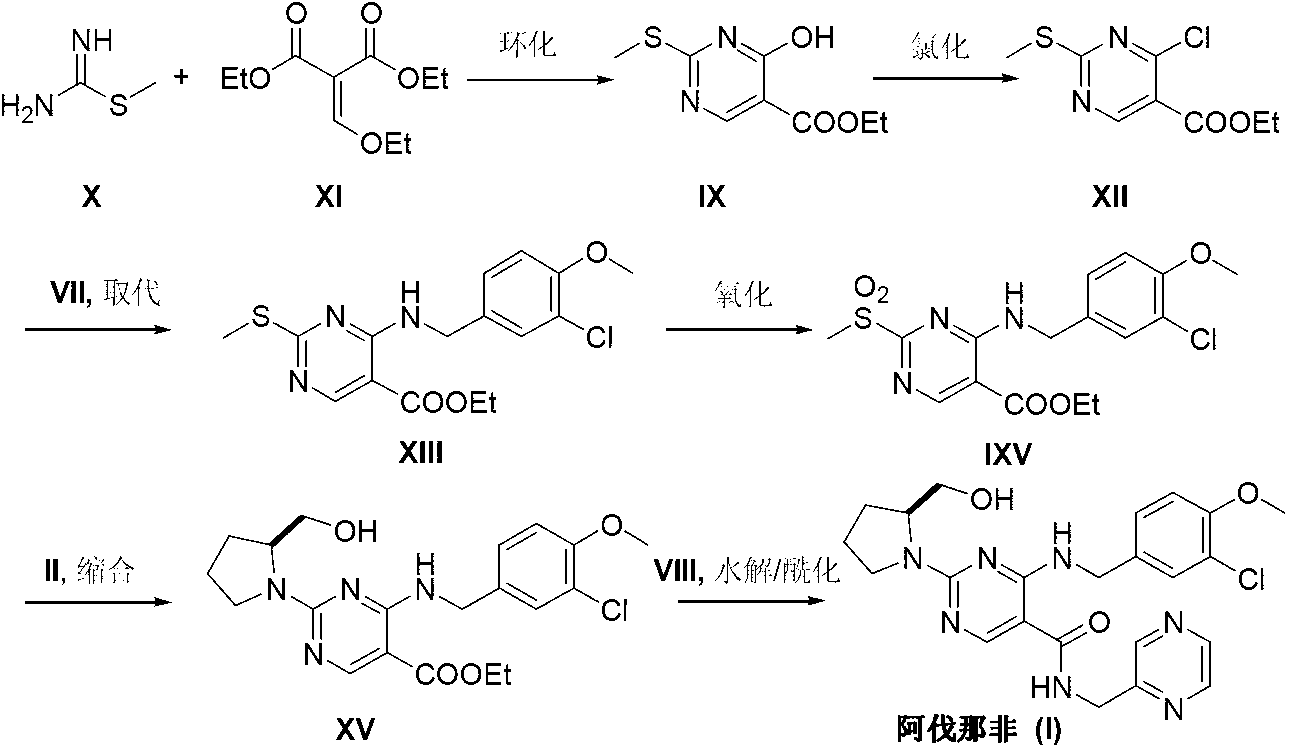
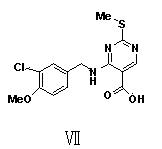
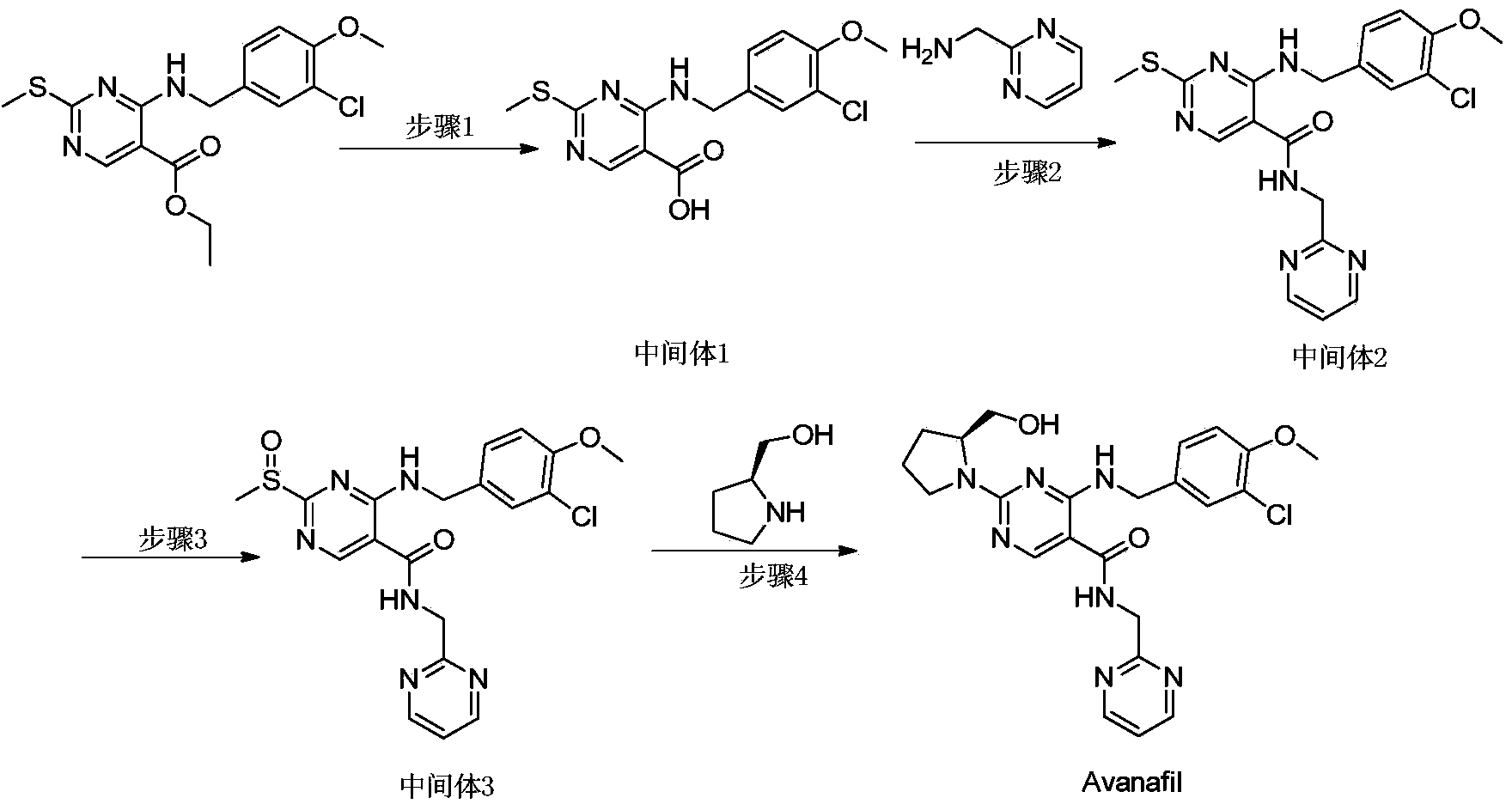
The invention provides a preparation method of avanafil, and specifically relates to the technical field of pharmaceutical chemistry. The preparation method of avanafil comprises the following steps:sequentially carrying out cyclization and chlorination reactions on methyl thiourea sulfuric acid and diethyl ethoxymethylene malonate which serve as initial raw materials to obtain 4-chloro-2-methylthiopyrimidine-5-carboxylic acid ethyl ester; then substituting and hydrolyzing with 3-chloro-4-methoxybenzylamine; condensing with 2-pyrimidinemethanamine to obtain a key intermediate, namely, 4-[(3-chloro-methoxybenzyl)amino]-2-methylthio-N-(2-pyrimidine methyl)-5-pyrimidine formamide; oxidizing the intermediate and reacting with L-prolinol to generate the avanafil. The preparation method has theadvantages of easy availability of raw materials, easiness and convenience in operation, mild reaction conditions and higher product yield.
Owner:苏州盛达药业有限公司
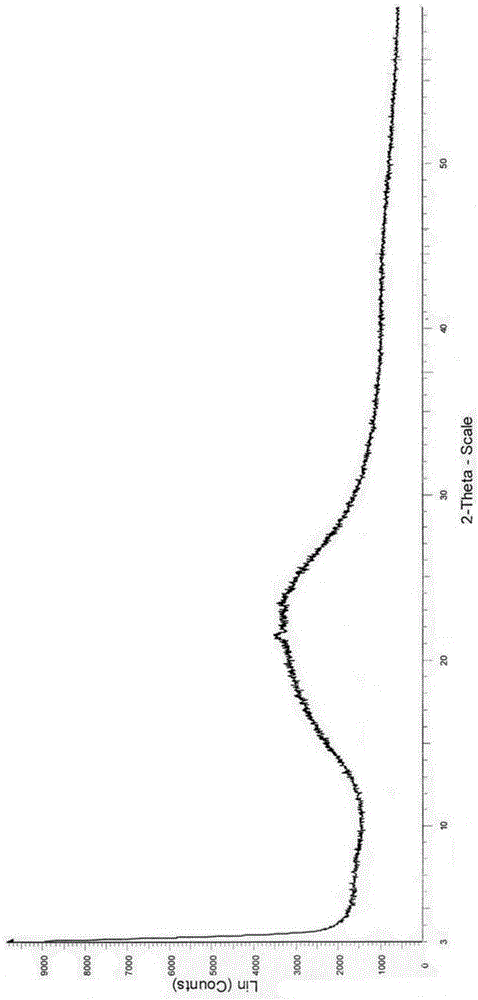
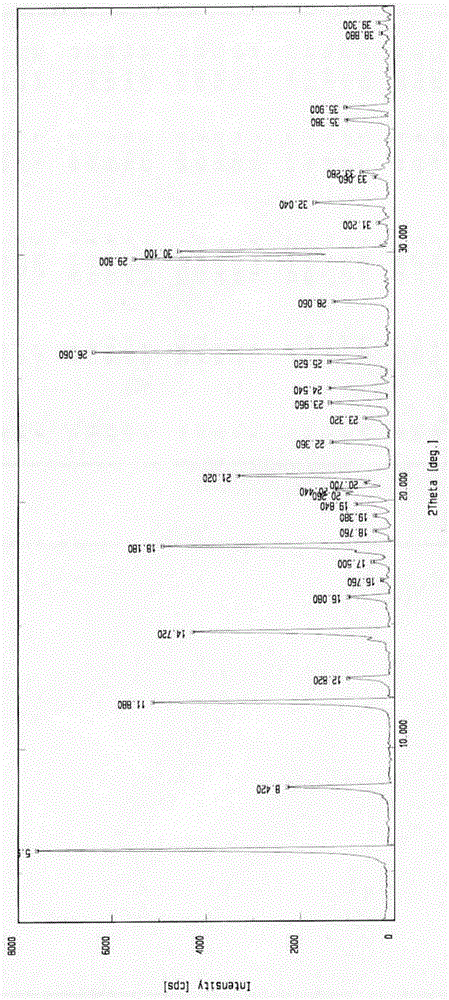
Amorphous form of avanafil, preparation method of, application and medicine composition of amorphous form of avanafil
InactiveCN104628707AStable in natureHigh purityOrganic active ingredientsOrganic chemistryMedicineDrugs preparations
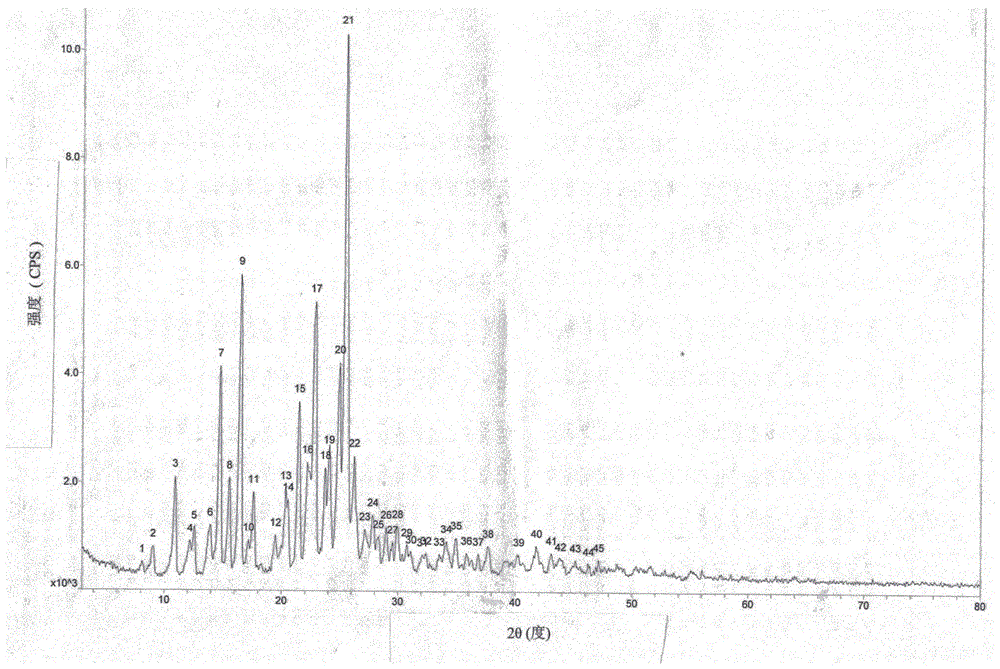
The invention relates to an amorphous form of avanafil, a preparation method, application and a medicine composition of the amorphous form of avanafil. The amorphous form of avanafil has no obvious characteristic peak in powder X-ray diffraction pattern based on Cu-K alpha radiation and shows a valuable characteristic in drug preparation; the amorphous form is high in purity and stable, and the in-vivo absorption of the amorphous form is superior to that of a crystal form.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Preparation method of avanafil important intermediate
InactiveCN105646240AReduce health hazardsRaw materials are cheap and easy to getOrganic compound preparationAmino-hyroxy compound preparation4-methoxybenzylamineAvanafil
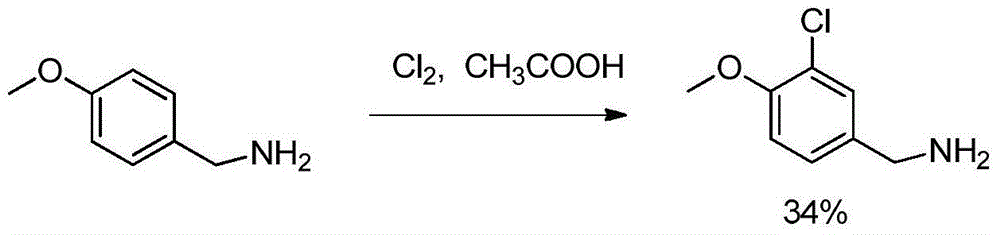
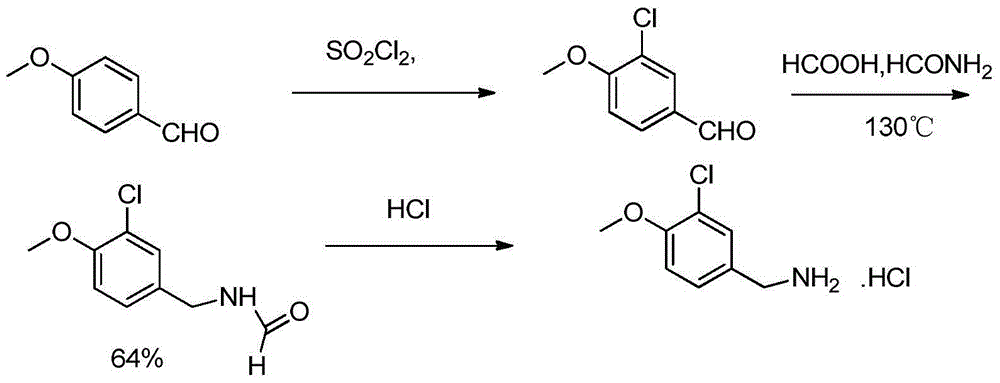
The invention provides a preparation method of an avanafil important intermediate. The target compound (3-chloro-4-methoxybenzylamine) is prepared according to a route shown as the specification. The preparation method provided by the invention has the advantages of cheap and easily available raw materials, short technical route, mild reaction conditions, low equipment requirement, and small health hazard to operators, and is in line with the idea of green chemistry. The method has high yield, and can obtain product with high purity, thus being suitable for industrial production.
Owner:重庆瑞泊莱医药科技有限公司
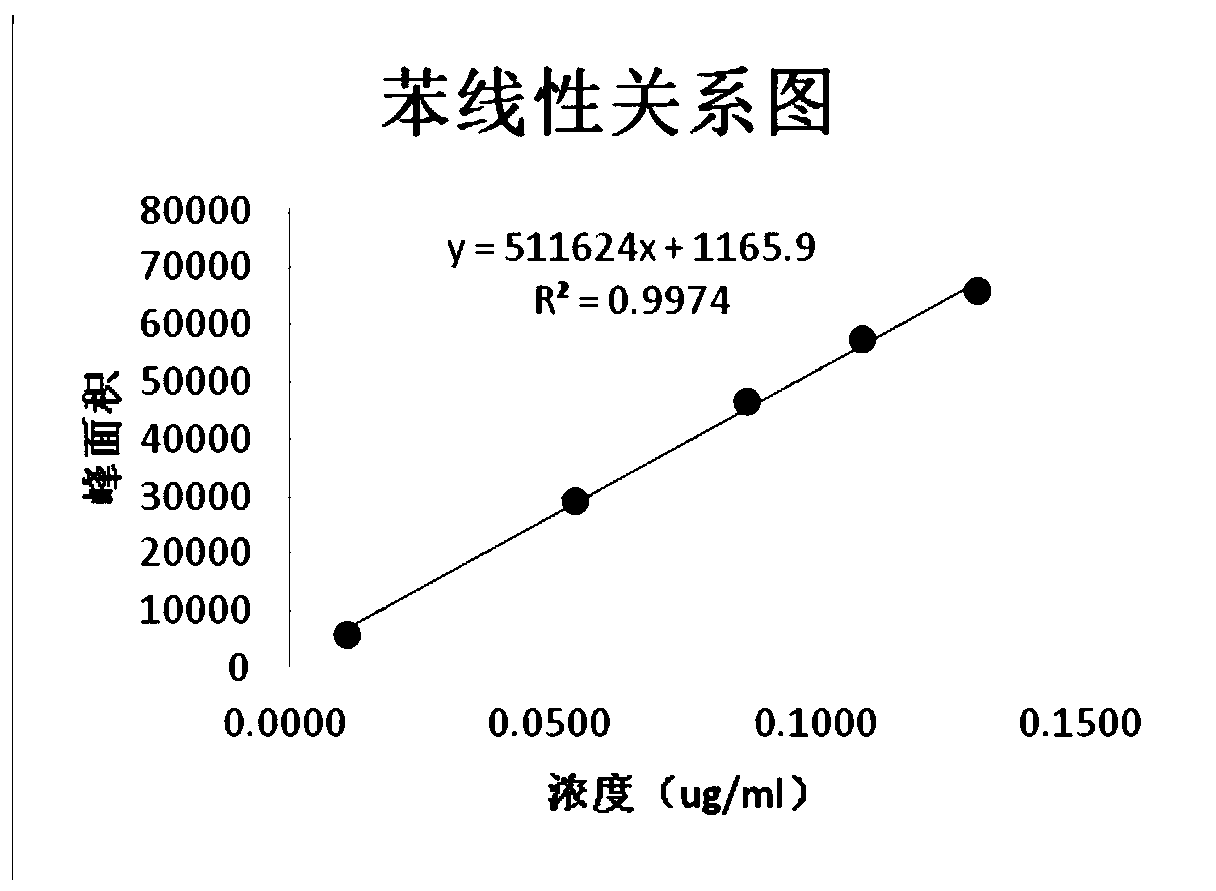
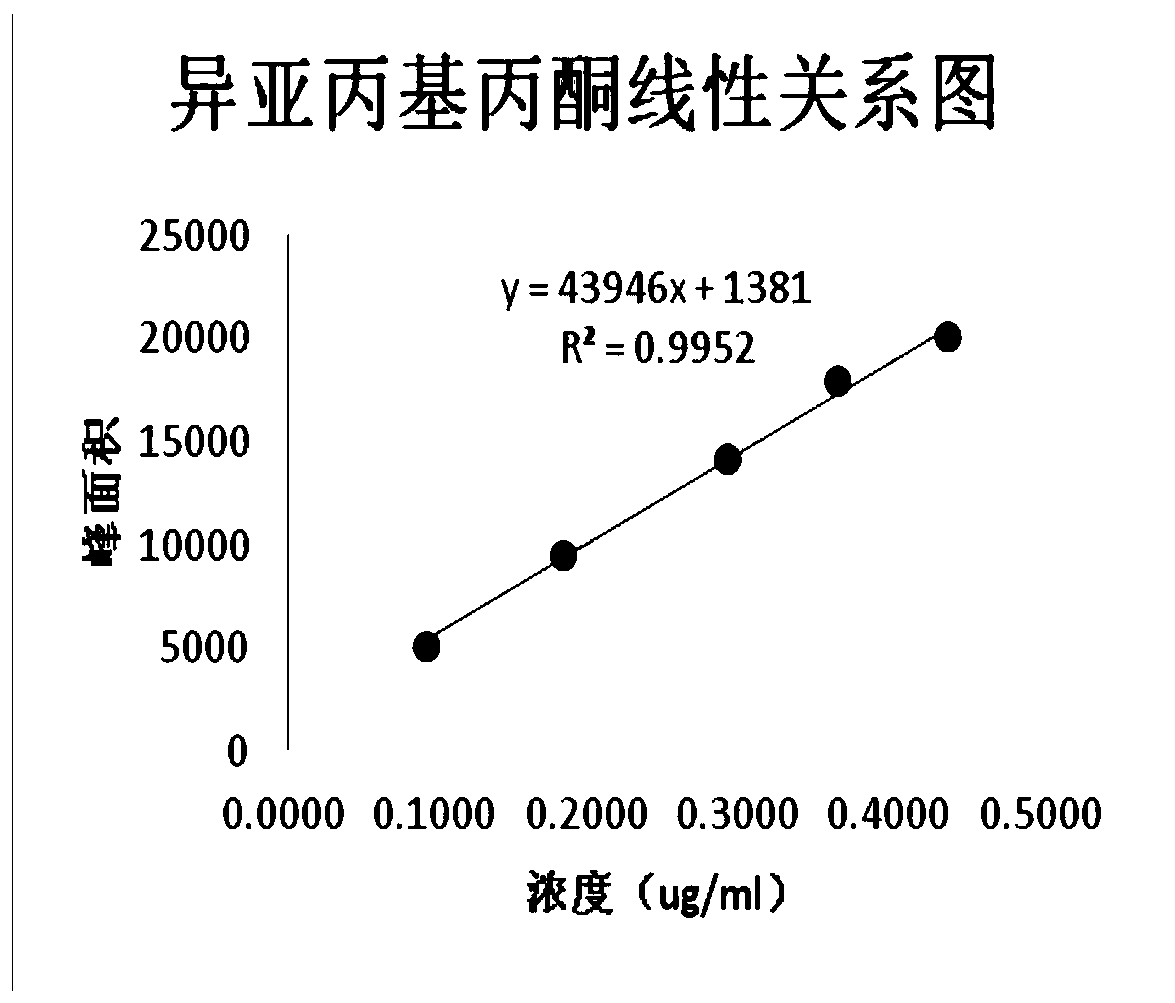
Method for detecting benzene and isopropylidene acetone residues in Avanafil by gas chromatography
The invention provides a method for detecting the content of residual solvent benzene and isopropylidene acetone in Avanafil, which comprises the steps of solution preparation and gas chromatography detection. The preparation the test sample solution includes the steps of weighing a test sample, placing the test sample in a volumetric flask, adding N- methyl pyrrolidone (NMP), strongly shaking todissolve, then diluting by water to a scale, and shaking well. The method has the advantages that the separation degree between each target peak to be detected and the adjacent impurity peak is high,there is no mutual interference, and accurate detection for benzene and isopropylidene acetone can be simultaneously realized. In addition, the method is simple and convenient to operate, easy to control and low in detection cost, and has good linear relationship, specificity, system applicability, sensitivity and high added sample recovery.
Owner:重庆瑞泊莱医药科技有限公司
Preparation method of avanafil intermediate
ActiveCN108658872AReduce dosageLow input costOrganic chemistryChemical recyclingCatalytic oxidationPorphyrin
The invention provides a preparation method of an avanafil intermediate. The preparation method comprises the following steps: dissolving a starting raw material 4-(3-chloro-4-methoxybenzylamino)5-ethoxycarbonyl-2-methylthiopyrimidine in a reaction solvent; adding metal porphyrin as a catalyst, thoroughly stirring for dispersing uniformly, and transferring to a reaction kettle; inflating an oxidizing gas at room temperature, and reacting while stirring to produce 4-(3-chloro-4-methoxybenzylamino) 5-ethoxycarbonyl-2-methylsulfinylpyrimidine. According to the preparation method, with substituentmetal porphyrin as the catalyst and air or oxygen as an oxidant, a sulfoxide intermediate of avanafil is prepared through catalytic oxidation of a thioether compound under a normal temperature condition; after a reaction, a target product is easily separated from the catalyst and has the characteristics of high yield, good purity and the like; the difficulty of separation and purification of theproduct in the later stage is greatly reduced; the recycled catalyst can be reused; the preparation cost can be significantly reduced.
Owner:无锡富泽药业有限公司
Orally disintegrating dosage form for administration of avanafil, and associated methods of manufacture and use
ActiveUS10028916B2Eliminates awkwardness and inconvenienceQuick releaseOrganic active ingredientsPill deliveryActive agentOrally disintegrating tablet
Owner:VIVUS LLC
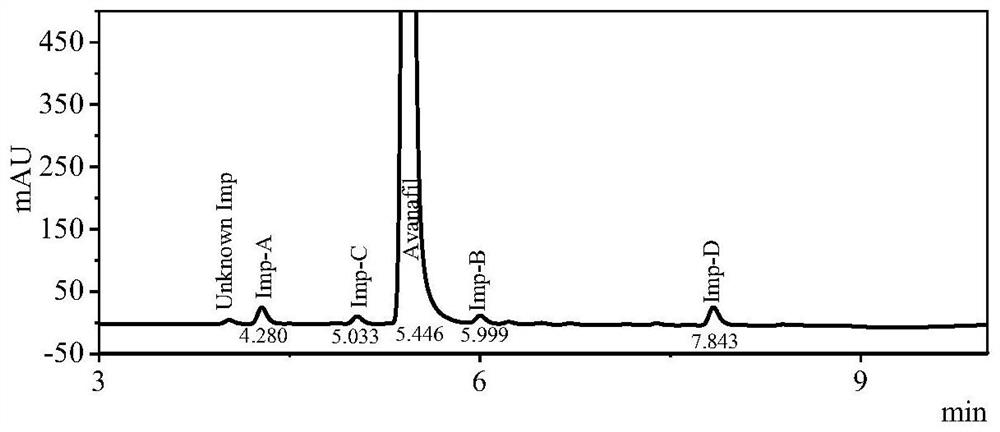
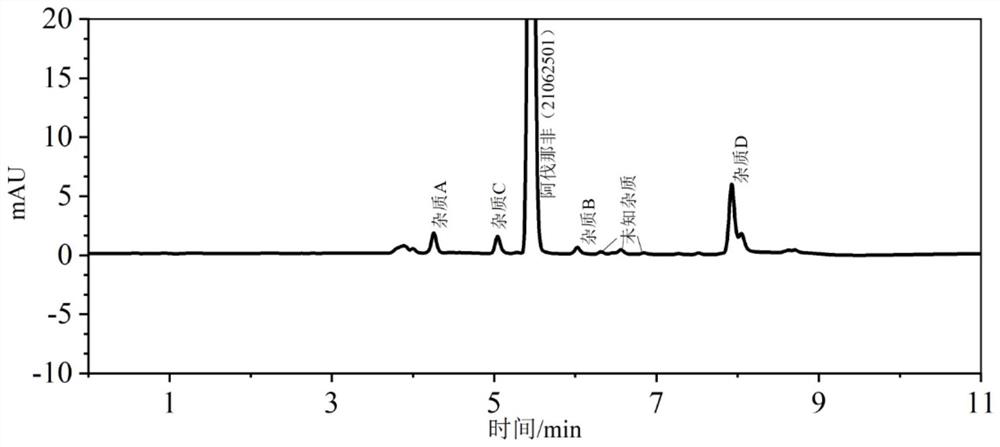
Method for analyzing related substances in avanafil and preparation thereof
PendingCN111208232AHigh sensitivityStrong specificityComponent separationUltraviolet detectorsPhosphate
The invention discloses a method for determining related substances in avanafil and a preparation thereof. The method is characterized in that a chromatographic column taking octyl bonded silica gel as a filler is adopted, phosphate buffer solution-acetonitrile is taken as a mobile phase, gradient elution is performed, and a diode array detector or an ultraviolet detector is adopted. The analysismethod is advantaged in that the avanafil and nine process impurities thereof can be separated, degraded impurities can further be effectively separated, and a blank solvent does not interfere with measurement, common instruments, equipment and the chromatographic column are used, the mobile phase is simple and easy to obtain, operation is simple and convenient, sensitivity is high, and the quality of avanafil and the preparation thereof can be better controlled.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Pharmaceutical composition for treating male erectile dysfunction and application thereof
ActiveCN104706639AReduce adverse reactionsExpand the scope of clinical applicationOrganic active ingredientsSexual disorderPenile TumescenceTreatment effect
The invention relates to a pharmaceutical composition for treating male erectile dysfunction and application thereof and belongs to the field of medicines. The invention provides a pharmaceutical composition with active ingredients containing avanafil by aiming at overcoming the defects that an existing medicine for treating the male erectile dysfunction has adverse effects and is short in action time or slowness for taking effect. The pharmaceutical composition can be used for obviously shortening an erection latent period and obviously prolonging an ejaculation latent period and has a good treatment effect on neuropathic ED.
Owner:NANJING ANGGU PHARMA TECH
Preparation method of Avanafil intermediate
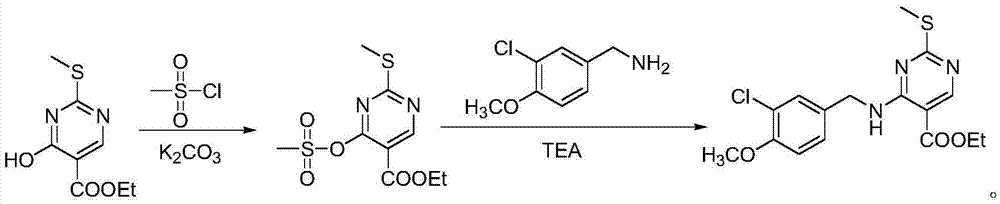
InactiveCN105439964AReduce dosageSafe post-processingOrganic chemistryEthyl esterMethanesulfonyl chloride
The invention provides a preparation method of an Avanafil intermediate. The preparation method comprises the following steps: dissolving 4-hydroxy-2-methylthio-ethyl 5-pyrimidinecarboxylate and potassium carbonate in DMF to obtain a mixed solution, adding methanesulfonyl chloride into the mixed solution under the stirring condition, continuously stirring after addition until a reaction is ended, adding water and ethyl acetate into a reaction solution, carrying out extraction and liquid separation, carrying out vacuum concentration on an organic phase to prepare 4-methanesulfonic sulfonic ester-2-methylthio-ethyl 5-pyrimidinecarboxylate; dissolving the obtained 4-methanesulfonic sulfonic ester-2-methylthio-ethyl 5-pyrimidinecarboxylate into DMF, adding a (3-chloro-4-methoxy)Benzylamine hydrochloride to obtain mixed liquor, adding triethylamine into the mixed liquor under the stirring condition, continuously stirring after addition until a reaction is ended, adding water and ethyl acetate, carrying out extraction and liquid separation, carrying out vacuum concentration on an organic phase, and recrystallizing to prepare 4-(3-chloro-4-methoxybenzylamino)-5- ethyloxycarbonyl-2-methylthiopyrimidine. The invention has advantages of less environmental pollution, high product yield, safe post-processing and simple operation.
Owner:HEBEI UNIVERSITY
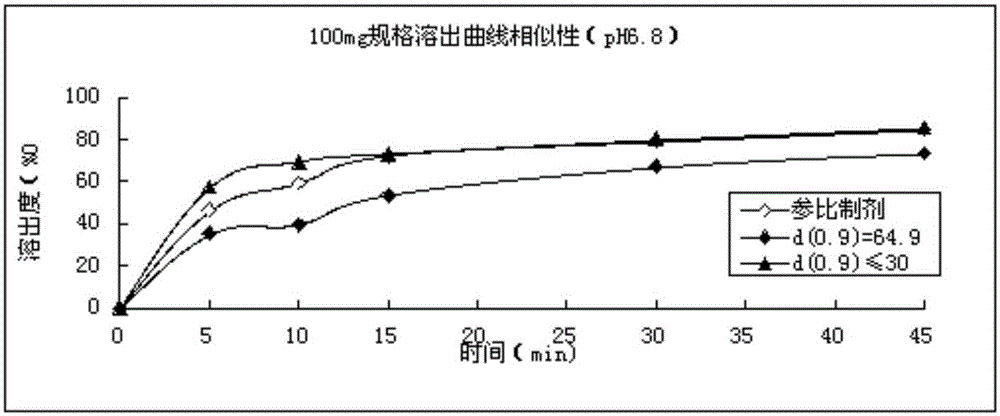
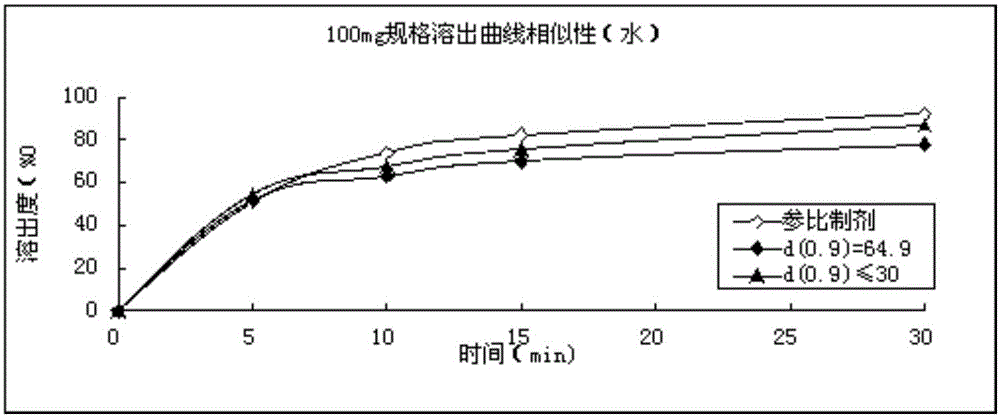
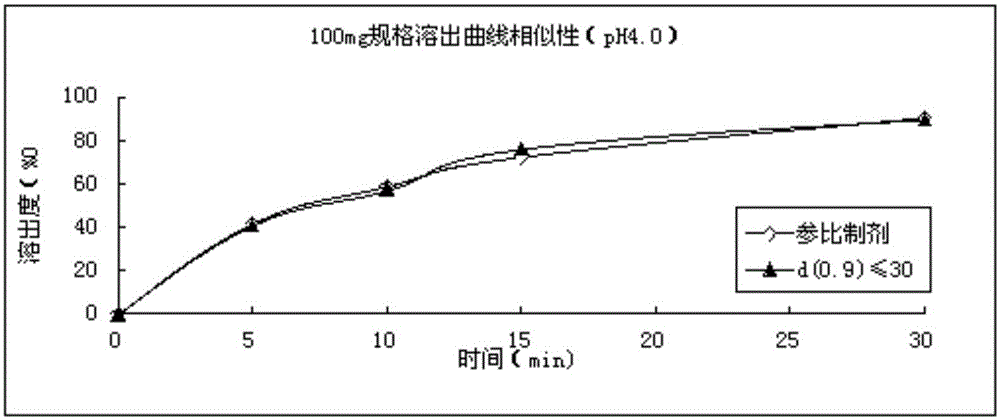
Refining method for avanafil
The invention discloses a refining method for avanafil. An avanafil bulk drug prepared by using the refining method has a particle size of no more than 30 [mu]m; so in the process of drying, an organic solvent can be easily volatilized and solvent residues are low in amount. The refining method comprises the following concrete steps: adding a crude avanafil product into an organic solvent and carrying out heating to a reflux state; after complete dissolving of solids, carrying out standing for cooling to 40 to 20 DEC C; then carrying out stirring for cooling to 10 to -10 DEG C and maintaining the temperature for 1 to 3 h; carrying out filtering; and subjecting a filter cake obtained in the previous step to vacuum drying. The organic solvent is a combination of two solvents, wherein one solvent is a strongly polar solvent and the other solvent is a moderately polar or non-polar solvent.
Owner:SHANDONG CHENGCHUANG PHARMA R&D
Avanafil preparation method
Disclosed is a method for preparing avanafil (I) by using cytosine as the starting material. The preparation steps comprise: using cytosine as the raw material, and enabling the cytosine to react with side chain 3-chlorine-4-methoxybenzyl halogen, N-(2-methylpyrimidine) methanamide and S-hydroxymethyl pyrrolidine, to prepare the target product avanafil (I). For the preparation method, the raw material is easily obtained, and the process is simple, economical, and environmentally friendly, so the method meets the requirement of industrial boost.
Owner:SUZHOU MIRACPHARMA TECH
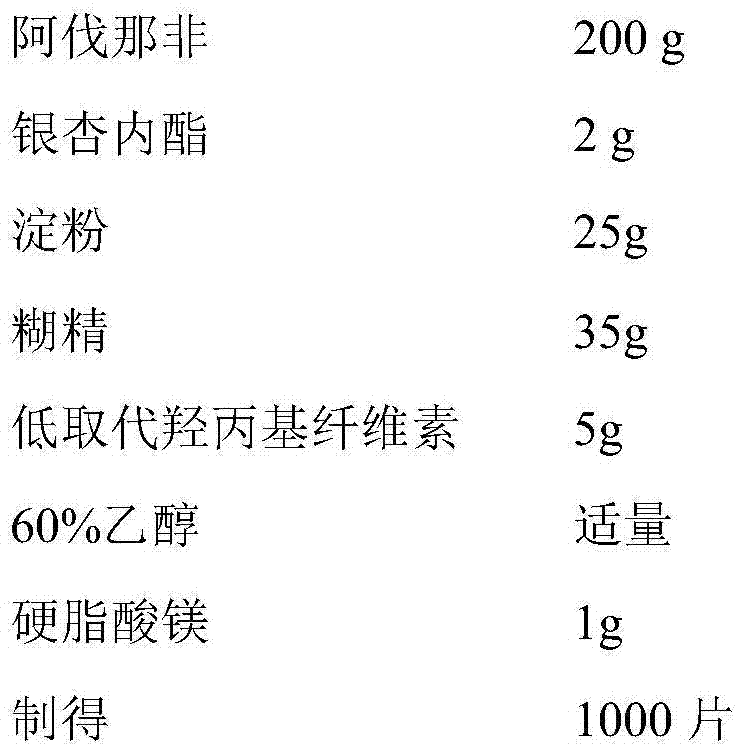
Avanafil preparation and preparation method thereof
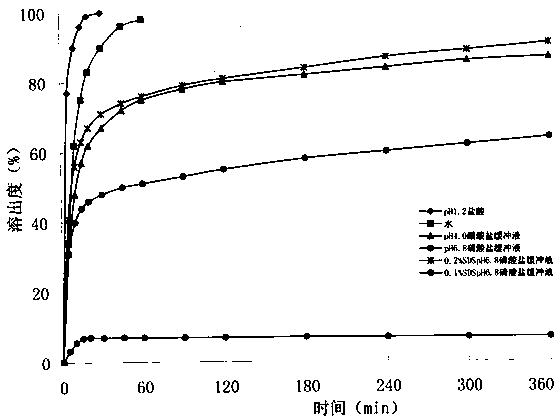
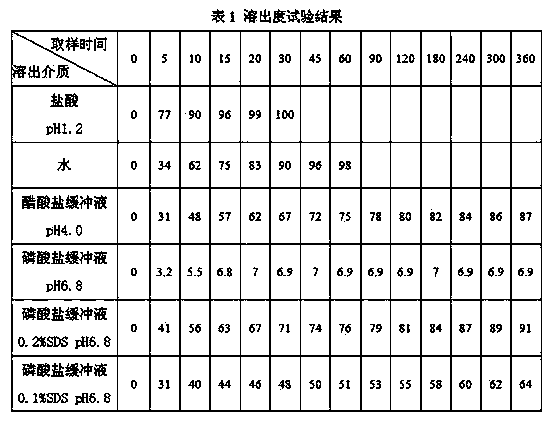
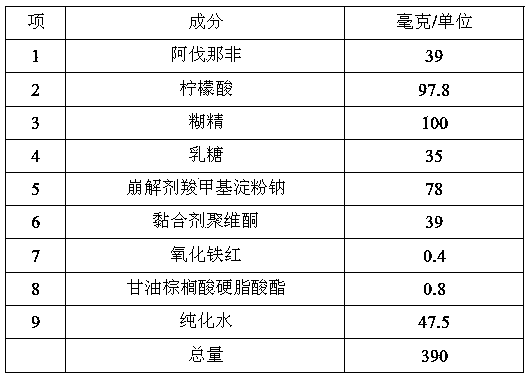
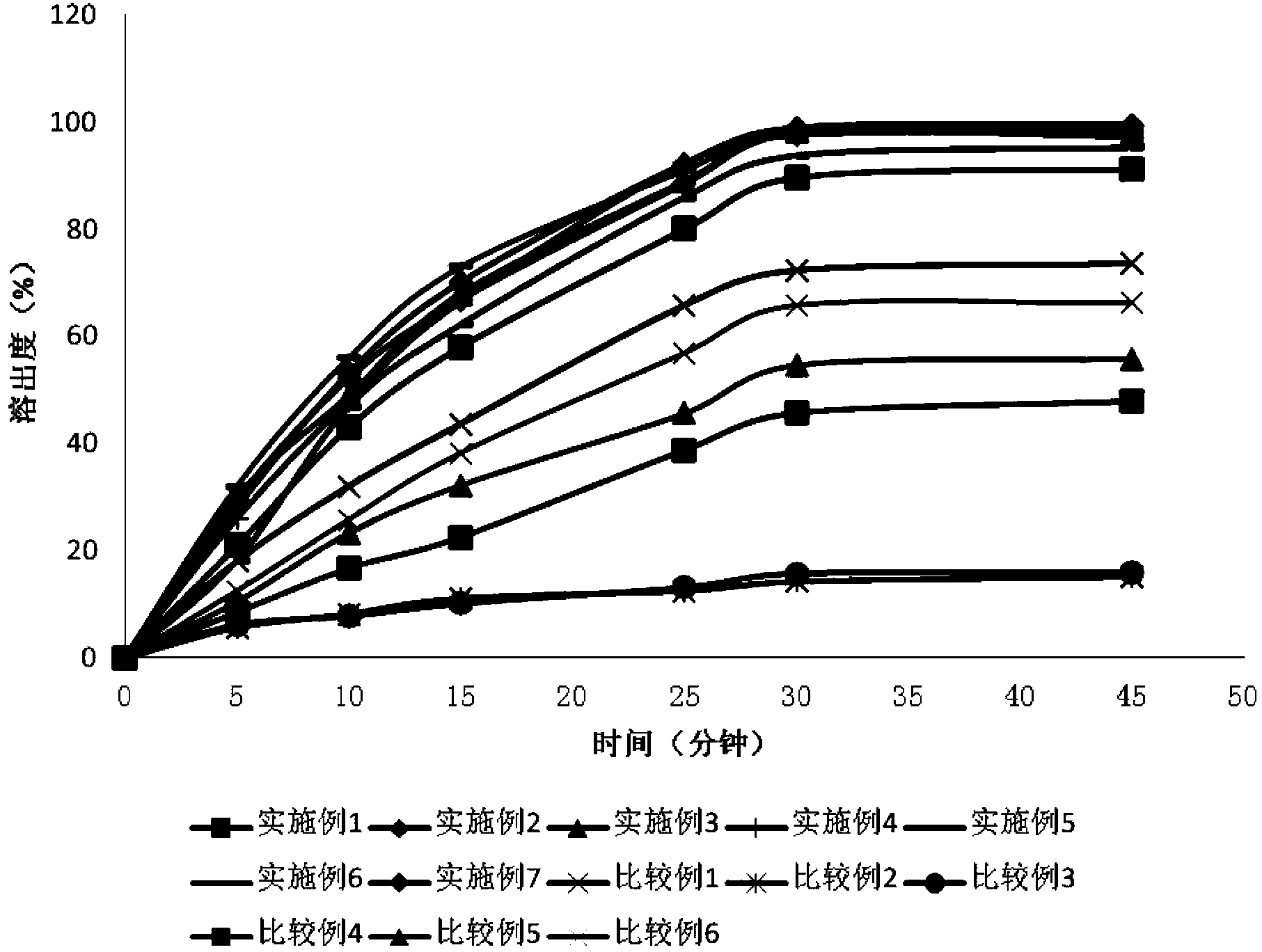
InactiveCN104188934AHigh dissolution rateHigh affinityOrganic active ingredientsPharmaceutical delivery mechanismAdhesiveCurative effect
The invention relates to an Avanafil preparation and a preparation method thereof. The Avanafil preparation consists of the following components in percentage by weight: 10-55% of Avanafil, 2-20% of a disintegrating agent, 18-25% of an acidifying agent, 0.2-10% of an adhesive, 0.2-1.0% of a lubricating agent, 0.1-0.5% of coloring agent and the balance of a filling agent; after raw auxiliary material mixing, wet process palletizing and drying, tablets are finally obtained. The preparation can be used for curing male erectile dysfunction and is taken approximately 30 minutes before sexual behavior, and the maximum frequency of dosage is one time a day. The preparation has good stability and dissolution rate, and also has the advantages of better curative effect, quicker action and lower dosage.
Owner:安徽联创生物医药股份有限公司
Development of an optimized avanafil-loaded invasomal transdermal film
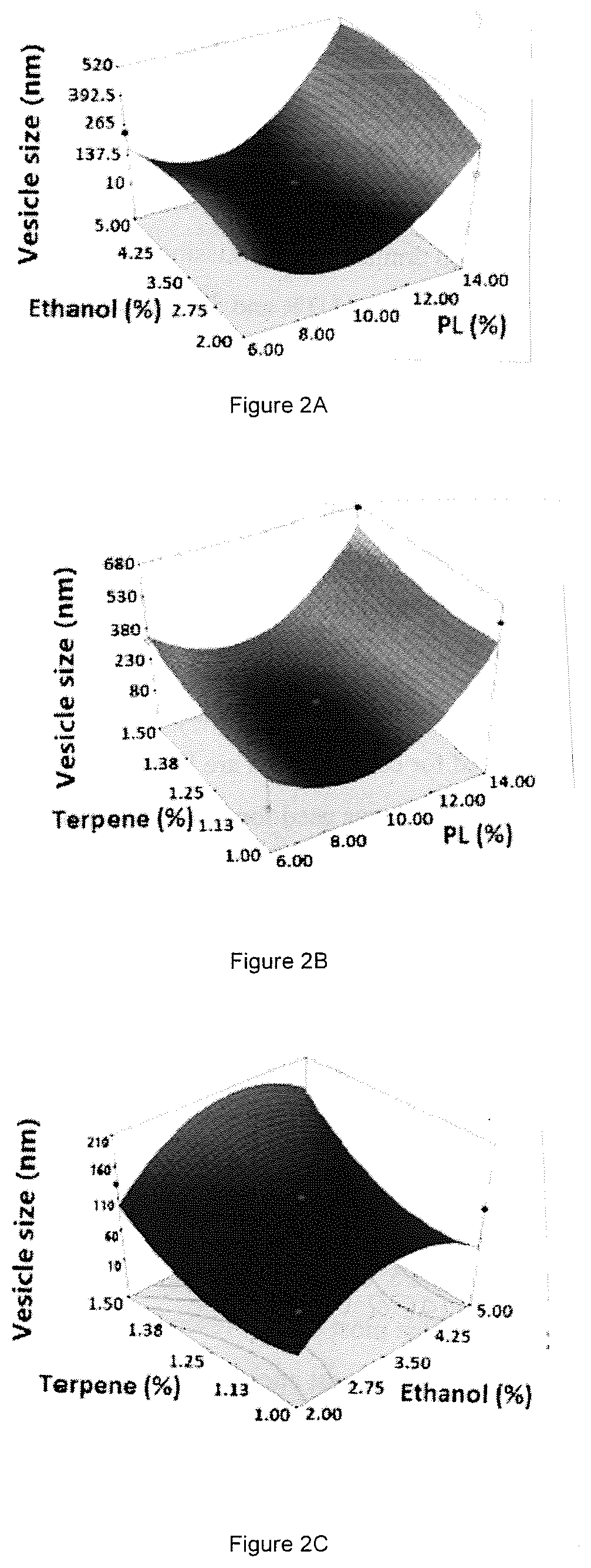
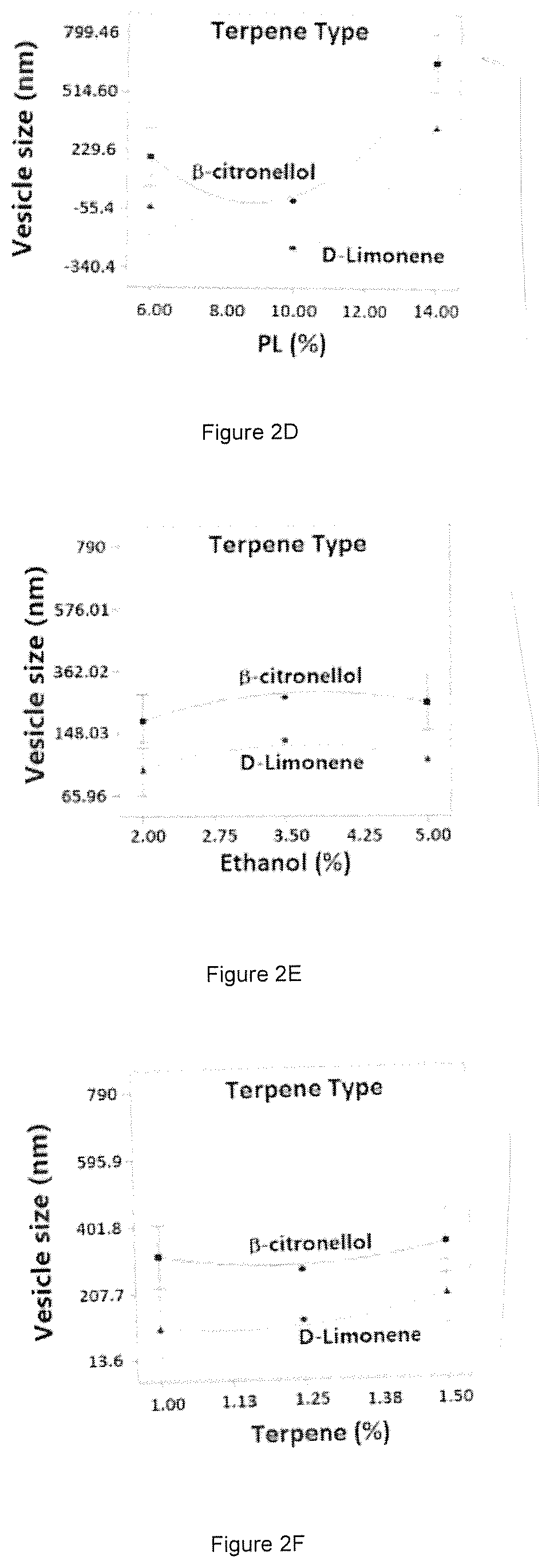
ActiveUS10751294B1Effective treatmentHigh encapsulation efficiencyOrganic active ingredientsSolution deliveryBiochemistryAvanafil
Nanosized avanafil (AVA) invasomes with enhanced transdermal delivery are provided. AVA invasomes were prepared with a vesicular size of 109.92 nm and an entrapment efficiency of 96.98%. The AVA invasomal film showed enhanced ex vivo permeation bioavailability compared to a raw AVA film.
Owner:KING ABDULAZIZ UNIV
Preparation method of avanafil
ActiveCN104530015AEasy to operateEase of industrial productionOrganic chemistryEthyl chloroformateSulfonyl chloride
The invention discloses a preparation method of avanafil. The method comprises the steps: carrying out amino protective reaction for cytosine (compound 1) which is used as a starting raw material and di-tert-butyl dicarbonate ester to obtain an intermediate I; enabling the intermediate I to firstly react with hexamethyl-disilazane and then to have friedel-crafts reaction with ethyl chloroformate in the existence of aluminum trichloride to obtain an intermediate II; preparing an intermediate III by virtue of substitution reaction between the intermediate II and 4-bromomethyl-2-chlorine-1-metoxybenzene; facilitating the reaction between the intermediate III and sulfonyl chloride to generate activated ester, and then enabling the activated ester to have condensation reaction with L-prolinol to obtain an intermediate IV, and carrying out the hydrolysis reaction for the intermediate IV to obtain an intermediate V; enabling the intermediate V to firstly react with CDI and then to have acylation reaction with 2-pyrimidinemethanamine oxalate to obtain avanafil. According to the method, raw materials for the reaction in each step are simple and easy to obtain, the operation is simple, the yield is high, the cost is low, and the industrialized production is easy to realize.
Owner:山东安信制药有限公司
Orally disintegrating dosage form for administration of avanafil, and associated methods of manufacture and use
ActiveUS20160331687A1Promote absorptionReduce aggregationOrganic active ingredientsPill deliveryActive agentOrally disintegrating tablet
Formulations are provided for the oral administration of avanafil, a Type V phosphodiesterase inhibitor (“PDE V inhibitor”), and analogs thereof. The formulations are orally disintegrating tablets (ODTs) that rapidly dissolve or disintegrate in the oral cavity. The tablets contain an absorption enhancing composition that increases the duodenal absorption of the active agent, following transfer from the low pH environment of the stomach to the more basic pH of the duodenum. Methods for administering the active agent using the dosage forms are provided. The invention also encompasses a method of selecting components and compositions to incorporate in the formulations which will facilitate increased absorption of the active agent in the duodenum and thus serve as “absorption enhancing compositions” herein. Also provided are methods for manufacturing orally disintegrating tablets to optimize the physical properties of the dosage forms, particularly hardness and disintegration time.
Owner:VIVUS LLC
Method for detecting avanafil and related impurities thereof
PendingCN114280174AMolecular weight determinationComponent separationPhysical chemistryMass Spectrometry-Mass Spectrometry
The invention discloses a method for detecting avanafil and related impurities thereof, ammonium formate-acetonitrile is taken as a mobile phase, and avanafil is analyzed and identified by adopting an analysis method of UPLC (Ultra Performance Liquid Chromatography) compatible with LC-MS (Liquid Chromatography-Mass Spectrometry), so that avanafil synthesized by adopting a specific synthesis route and related impurities thereof can be effectively separated; and a new method is provided for key process parameter control and quality control of the avanafil bulk drug.
Owner:HARVEST PHARMA HUNAN CO LTD
Avanafil effervescent dry suspension and preparation method thereof
InactiveCN104248625AHigh dissolution rateOvercome the defect of low dissolution rateOrganic active ingredientsPowder deliveryDissolutionSuspending Agents
The invention provides an avanafil effervescent dry suspension and a preparation method thereof. The avanafil effervescent dry suspension comprises avanafil, an effervescent disintegrant, a filler, a suspending agent and other optional medicinal auxiliary materials, wherein the other medicinal auxiliary materials are one or more of a flow aid, a sweetener, essence and a colouring agent, the effervescent disintegrant is composed of an acidic substance and an alkali substance, the weight ratio of avanafil to the acidic substance is 1:(6-25), and the weight ratio of the acidic substance to the alkali substance is 2.5:1 or more. The avanafil effervescent dry suspension is rapid to disperse, fast in effectiveness, high in bioavailability and good in stability, and has the avanafil dissolution rate up to 89% or more.
Owner:NEW FOUNDER HLDG DEV LLC +2
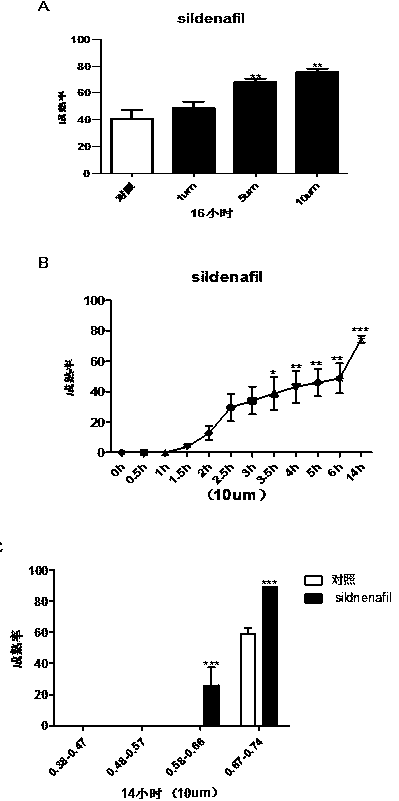
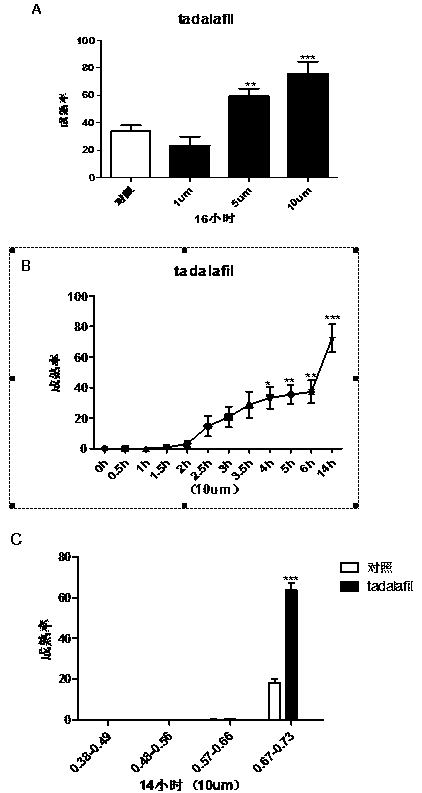
Application of phosphodiesterase 5 activity inhibitor as ripening agent for inducing maturation of zebrafish oocyte
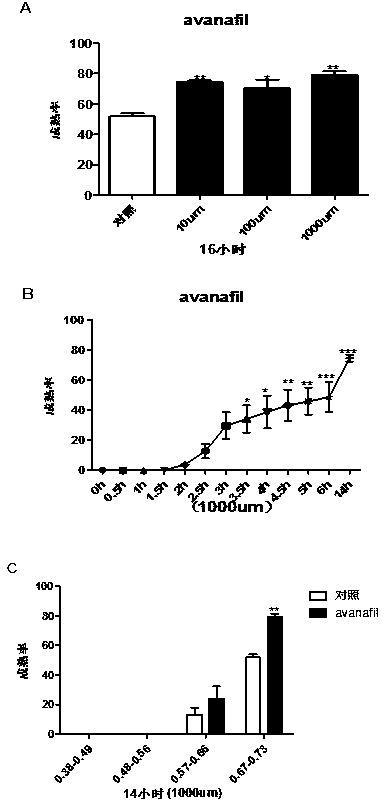
The present invention provides a novel use of a phosphodiesterase 5 activity inhibitor, namely, as a ripening agent for inducing maturation of zebrafish oocyte, which belongs to the technical field ofaquatic products. The phosphodiesterase 5 activity inhibitor comprises Sildenafil Citrate, Tadalafil and Avanafil. The experiment shows that Sildenafil Citrate, Tadalafil and Avanafil exhibit a dose-dependent, time-dependent and period-dependent manner in promoting oocyte maturation, and moreover, the efficiency of inducing oocyte maturation in zebrafish is higher. In addition, the phosphodiesterase 5 activity inhibitor can be used not only for inducing maturation of zebrafish oocyte, but also for inducing maturation of other fish oocytes, so that the phosphodiesterase 5 activity inhibitor has good application prospect.
Owner:NORTHWEST NORMAL UNIVERSITY
Compound, crystal form compound and preparation method thereof
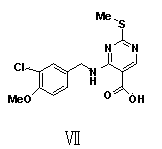
ActiveCN104151299BHigh purityLow impurity contentCarboxylic acid salt preparationAvanafilMedicinal chemistry
Owner:BEIJING COLLAB PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com