Avanafil preparation and preparation method thereof
A technology of avanafil and preparation, which is applied in the field of phosphodiesterase-5 inhibitor and its preparation, can solve the problems of low dissolution rate, affecting drug bioavailability and curative effect, etc., so as to increase affinity and improve bioavailability , quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
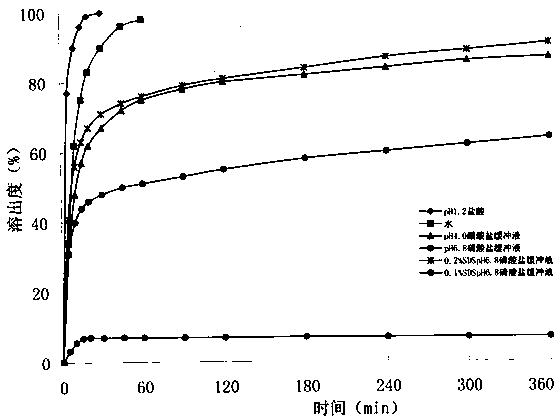
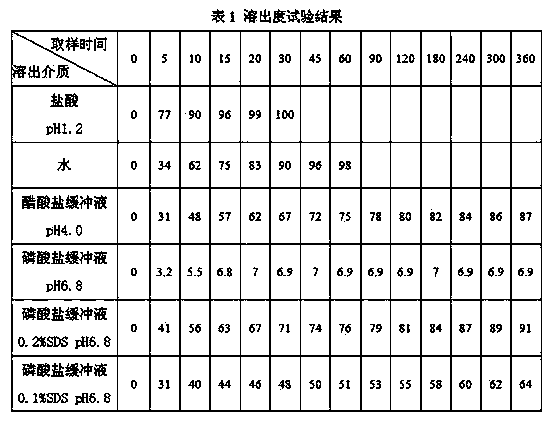
Image
Examples
Embodiment 1
[0040] This embodiment relates to an avanafil preparation and a preparation method thereof.
[0041] Step (1) Ingredients:
[0042]
[0043] Step (2) Take avanafil, grind it, pass through a 100-mesh sieve, and pass the above-mentioned amounts of fumaric acid, calcium carbonate, mannitol, and low-substituted hydroxypropyl cellulose through a 60-mesh sieve;
[0044] Step (3) Mix the above-mentioned raw and auxiliary materials evenly, granulate the above-mentioned amount of 5% (w / w) soft material made of hydroxypropyl cellulose, sieve 25 mesh, dry at 41°C, granulate with 24 mesh, and then add the above-mentioned Quantities of magnesium stearate and iron oxide yellow are mixed and pressed into tablets;
[0045] Step (4) Use water as a solvent, prepare a film coating premix solution with a solid content of 25% for coating, and the coating weight accounts for 2% of the weight of the entire tablet.
Embodiment 2
[0047] This embodiment relates to an avanafil preparation and a preparation method thereof.
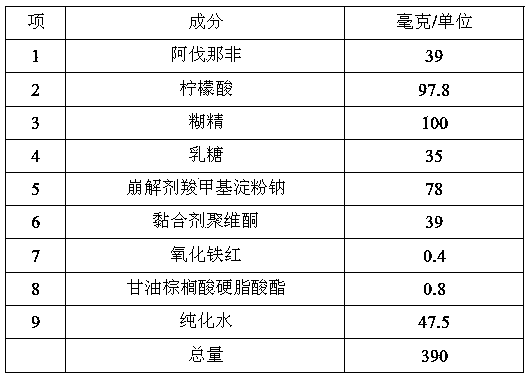
[0048] Step (1) Ingredients:
[0049]
[0050] Step (2) Take avanafil, pulverize it, pass through a 120-mesh sieve, and pass the above-mentioned quantities of citric acid, dextrin, lactose, and sodium carboxymethyl starch through a 60-mesh sieve for later use;
[0051] Step (3) Mix the above-mentioned raw and auxiliary materials evenly, granulate the above-mentioned 5% (w / w) binder soft material made of povidone, sieve 20 mesh, dry at 50°C, granulate at 25 mesh, and then add the above-mentioned Amount of glycerol palmitostearate, red iron oxide mixed, pressed into tablets;
[0052] Step (4) Use water as a solvent to prepare a film coating premix solution with a solid content of 15% for coating test. The coating weight accounts for 3% of the weight of the entire tablet, and the tablet is obtained.
Embodiment 3
[0054] This embodiment relates to an avanafil preparation and a preparation method thereof.
[0055] Step (1) Ingredients:
[0056]
[0057] Step (2) Take avanafil, pulverize it, pass through a 100-mesh sieve, and pass the above-mentioned amounts of adipic acid, microcrystalline cellulose, calcium hydrogen phosphate, and disintegrating agent crospovidone through a 40-mesh sieve;
[0058] Step (3) Mix the above-mentioned raw and auxiliary materials evenly, granulate the above-mentioned amount of 5% (w / w) adhesive starch soft material, sieve 24 mesh, dry at 55°C, granulate at 20 mesh, and then add the above-mentioned amount of Mix polyethylene glycol and edible yellow pigment evenly, and press into tablets;
[0059] Step (4) Use water as a solvent, prepare a film coating premix solution with a solid content of 20%, and conduct a coating test. The coating weight accounts for 5% of the weight of the entire tablet, and the product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com