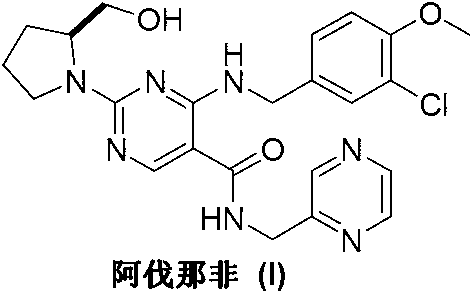
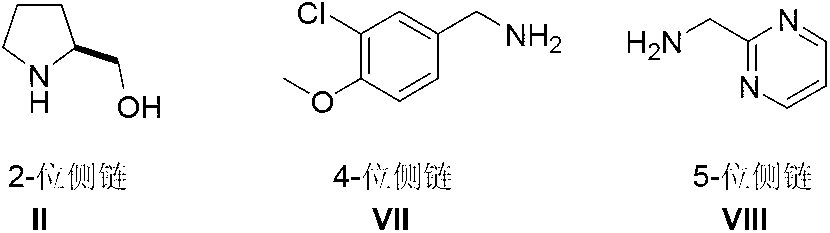
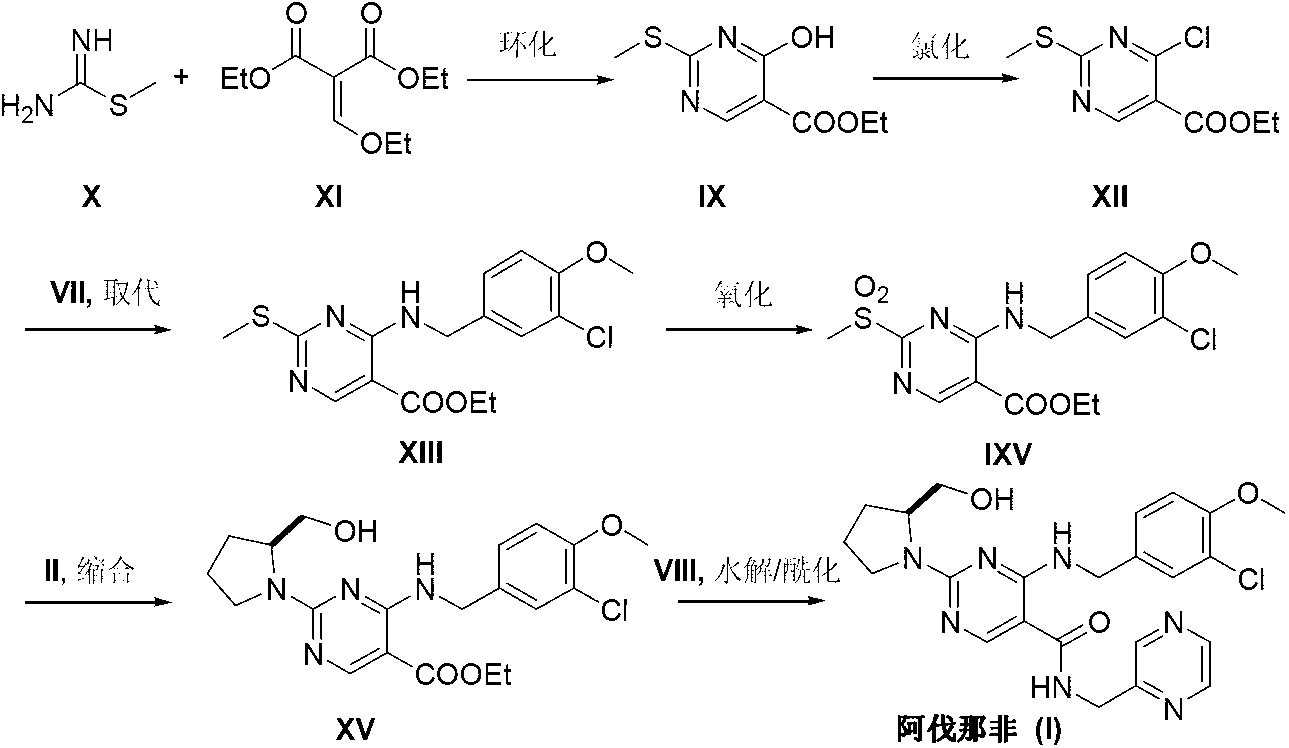
Preparation method of Avanafil
A technology of avanafil and methoxybenzyl, which is applied in the field of preparation of avanafil, can solve problems such as difficult separation, difficulty in realizing industrialization, complex process, etc., and achieve the effect of simple process and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add cytosine (2.22g, 20mmol), triethylamine (2.0g, 20mmol), potassium iodide (0.2g, 1% eq) and 50mL of absolute ethanol into the three-neck flask, raise the temperature to 50-55°C, and stir until the system dissolves Uniform. 3-Chloro-4-methoxybenzyl bromide (III) (5.60 g, 24 mol) was slowly added dropwise into the reaction liquid. The temperature was raised to 80° C., and the reaction was continued for 3 hours, and the reaction was detected by TLC. Cool down to room temperature, and remove triethylamine hydrobromide by filtration. The filtrate was adjusted to pH 4-5 with hydrochloric acid. Ethanol was recovered under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain 4.78 g of off-white solid N-(3-chloro-4-methoxybenzyl)cytosine (V), with a yield of 90.2%.
Embodiment 2
[0032] Under nitrogen protection, N-(3-chloro-4-methoxybenzyl)cytosine (V) (2.65g, 10mmol), benzotriazol-1-yloxytri(di Methylamino)phosphonium hexafluorophosphate (BOP) (6.63 g, 15 mmol) and acetonitrile 50 mL. Under stirring, 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) (2.28 g, 15 mmol) was added dropwise, and the reaction was carried out at room temperature for 12 hours. The temperature was raised to 60° C., and the reaction was continued for 12 hours. The solvent was distilled off under reduced pressure, dissolved in 100 mL of ethyl acetate, and washed with 20 mL of 2M sodium hydroxide. The organic phase was separated, dried and concentrated under reduced pressure. The residue was dissolved in 100 mL of tetrahydrofuran, S-hydroxymethylpyrrolidine (II) (1.31 g, 13 mmol) and sodium hydride (0.32 g, 13 mmol) were added, the temperature was raised to 55°C, and the reaction was stirred for 5 hours, and the reaction was monitored by TLC. The reaction was quenched with saturated ...
Embodiment 3
[0034]Add 4-[(3-chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]pyrimidine (VI) (1.74 g, 5mmol), liquid bromine (0.94g, 6mmol) and 25mL of 2.0M sodium hydroxide solution, 200W microwave irradiation for 5 minutes, cooled to room temperature, a white solid precipitated. After filtering and drying, add 25 mL of ethylene glycol dimethyl ether to dissolve and transfer to a three-necked reaction flask. Add nickel acetate tetrahydrate (13 mg, 0.05 mmol), tris(2,4-di-tert-butyl)phenoxyphosphine (32 mg, 0.05 mmol), sodium methoxide (0.54 g, 10 mmol) and N-(2-methyl Pyrimidine) formamide (IV) (2.05 g, 15 mmol), under the protection of nitrogen, the temperature was raised to 120° C., the reaction was stirred for 10 hours, and the reaction was detected by TLC. The reaction solution was poured into 25 mL of saturated ammonium chloride solution, and extracted three times with ethyl acetate. The organic phases were combined, dried over anhydrous magnesium sulfate, the sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com