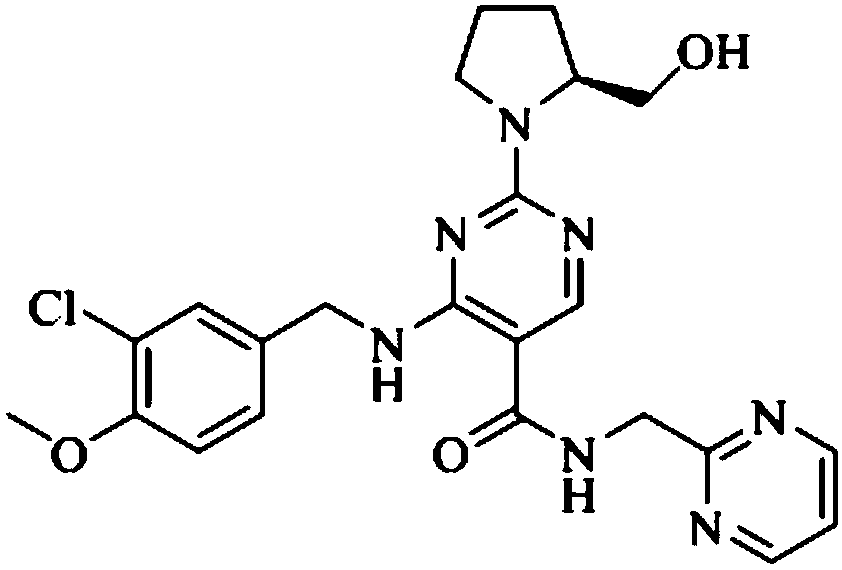
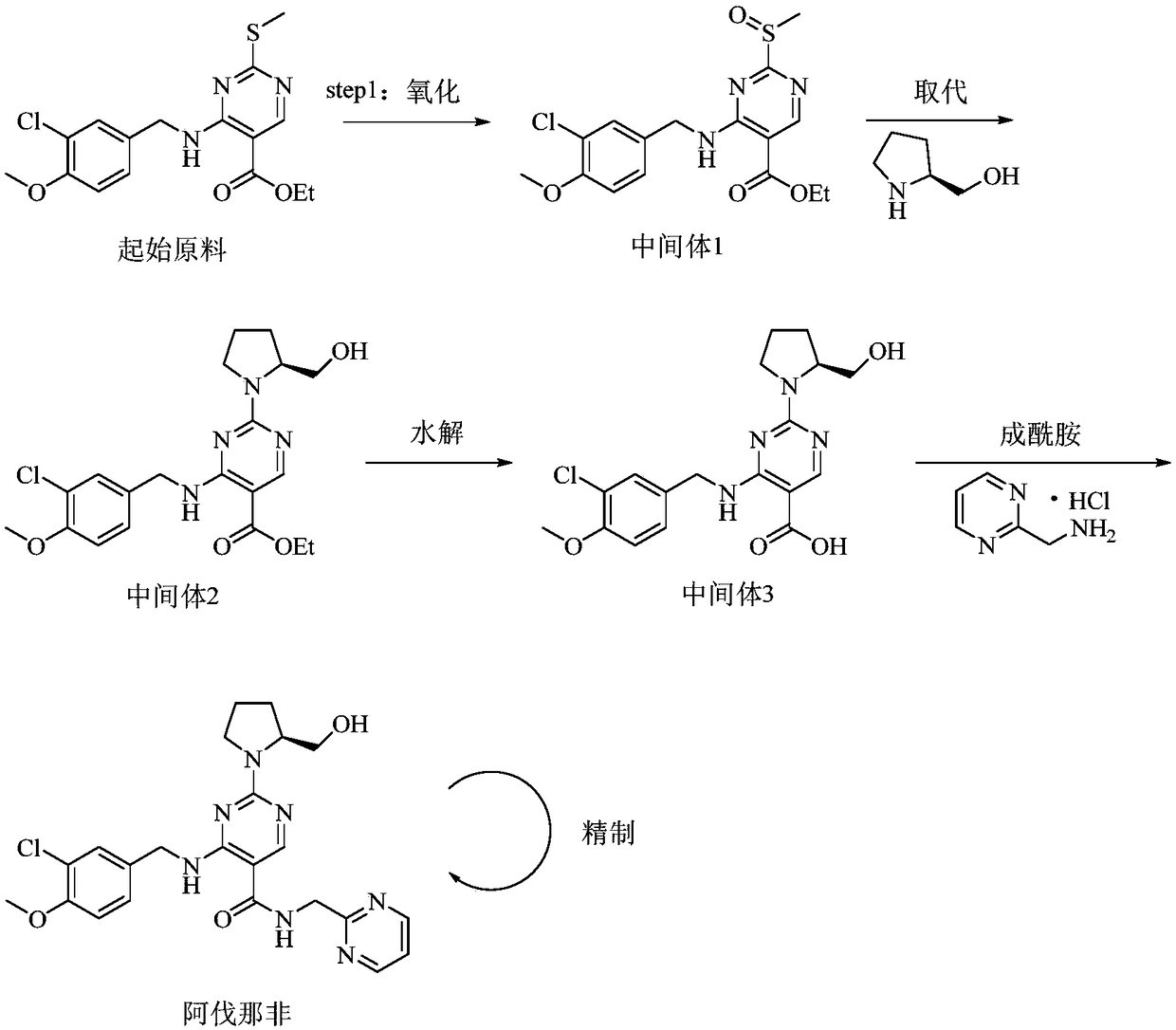
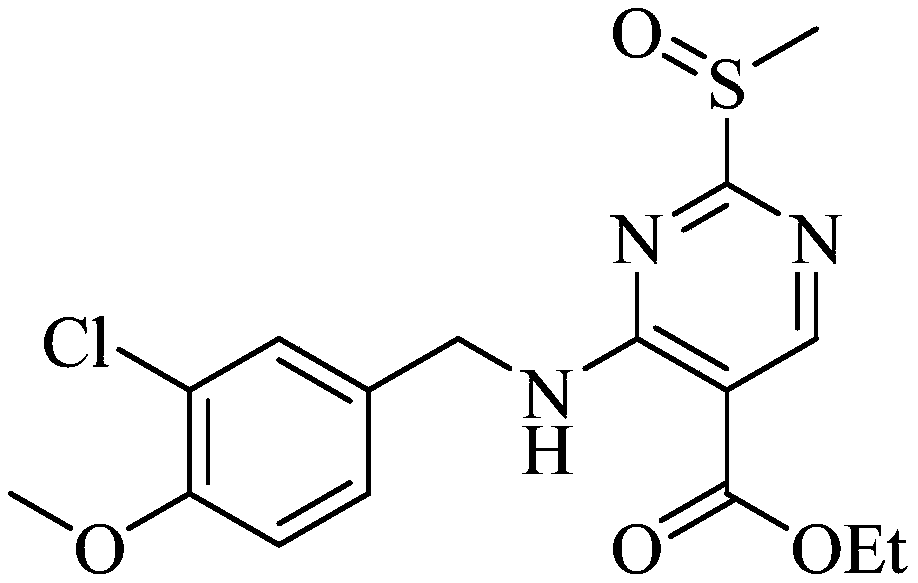
Preparation method of avanafil intermediate
A technology of avanafil and intermediates, applied in the field of pharmaceutical chemical synthesis, can solve the problems of low purity of m-CPBA chemicals, unsuitability for large-scale industrialization, and increased difficulty of post-processing, etc. Industrialized production, reduced manpower, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 4-(3-Chloro-4-methoxybenzylamino) 5-ethoxycarbonyl-2-methylthiopyrimidine (36.8g, 100mmol), ascorbic acid (52.8g, 300mmol) were dissolved in 500mL toluene, added Iron tetraphenylporphyrin (0.67g, 1mmol) was fully stirred to disperse evenly, and transferred to a pressurized reactor. Air was introduced at 25°C, the reaction pressure was adjusted and controlled at 1.0 atm, and the reaction was stirred for 8 hours. After the reaction, the metalloporphyrin catalyst in the reaction solution was removed by filtration, and the organic phase was washed with purified water and saturated NaCl once, and concentrated under reduced pressure to obtain a white thick liquid, which was the avanafil intermediate, 4-(3 -Chloro-4-methoxybenzylamino)5-ethoxycarbonyl-2-methylsulfinylpyrimidine 36.47g (95.0% yield, 99.3% purity) was directly used in the next reaction.
[0039] IR(neat)cm -1 :3350,1695,1590,1570,1500,1460,1440
[0040] MS(m / z):384(M+H) +
Embodiment 2
[0042] Dissolve 4-(3-chloro-4-methoxybenzylamino)5-ethoxycarbonyl-2-methylthiopyrimidine (36.8g, 100mmol), isobutyraldehyde (7.2g, 100mmol) in 1000mL acetic acid Ethyl ester was added manganese tetraphenylporphyrin (0.33g, 0.5mmol) and stirred well to disperse evenly, and then transferred to a pressurized reactor. Oxygen was introduced at 25° C., the reaction pressure was adjusted and controlled at 5.0 atm, and the reaction was stirred for 16 hours. After the reaction, the metalloporphyrin catalyst in the reaction solution was removed by filtration, the organic phase was washed with purified water and saturated NaCl once, and concentrated under reduced pressure to obtain a white thick liquid, which was the avanafil intermediate, 4-( 35.05 g of 3-chloro-4-methoxybenzylamino)5-ethoxycarbonyl-2-methylsulfinylpyrimidine (91.3% yield, 98.0% purity) was directly used in the next reaction.
Embodiment 3
[0044]Dissolve 4-(3-chloro-4-methoxybenzylamino)5-ethoxycarbonyl-2-methylthiopyrimidine (36.8g, 100mmol), propionaldehyde (29.0g, 500mmol) in 100mL tetrahydrofuran, Add cobalt tetraphenylporphyrin (0.07 g, 0.1 mmol) and stir well to make the dispersion uniform, and transfer to a pressurized reactor. Air was introduced at 25°C, the reaction pressure was adjusted and controlled at 2.5 atm, and the reaction was stirred for 20 hours. After the reaction was completed, the metalloporphyrin catalyst in the reaction solution was removed by filtration, and the reaction solution was concentrated under reduced pressure to dryness. Add 500mL ethyl acetate and stir to dissolve, the organic phase is washed with purified water and saturated NaCl once, and concentrated under reduced pressure to obtain a white thick liquid, which is the intermediate of avanafil, 4-(3-chloro-4-methyl Oxybenzylamino) 5-ethoxycarbonyl-2-methylsulfinylpyrimidine 34.05g (yield 88.7%, purity 98.6%) was directly use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com