Novel intermediate used for preparation of avanafil and preparation method thereof
A technology of avanafil and intermediates, which is applied in the field of key intermediates of avanafil, can solve the problems of cumbersome post-processing, etc., and achieve the effect of being beneficial to purification, easy to purify, and reducing the use of column chromatography
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
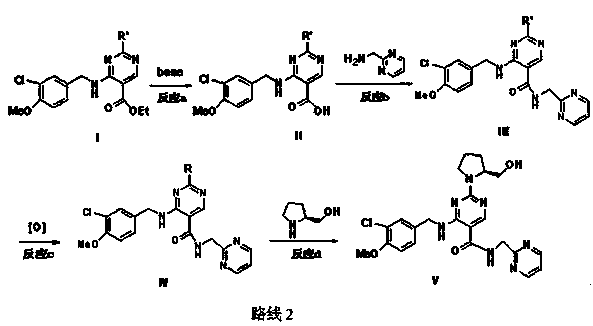
[0035] (1) Preparation of 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine (Ⅵ):
[0036]
[0037] Add 15g of 4-chloro-5-ethoxycarbonyl-2-methylthiopyrimidine and 18ml of triethylamine to dissolve in 130ml of tetrahydrofuran respectively, cool down to 0°C, and add 13.5g of 3-chloro-4-methoxy Benzylamine, react at 0-5°C for 30 minutes, then react at 20-30°C, and detect the end point of the reaction by HPLC. The reactant was concentrated under reduced pressure, and 120 ml of ethyl acetate and citric acid aqueous solution were added for washing. The organic phase was washed with water and brine, dried, filtered, and concentrated under reduced pressure to obtain 22 g of crude product. Finally, dissolve the crude product with absolute ethanol at 55-60°C, stir and crystallize overnight, filter, and dry to obtain 15 g of the product. 1 H NMR: 8.78 (t, J =6.0Hz, 1H), 8.55(s, 1H), 7.43(s, 1H), 7.29(d, J =8.4Hz, 1H), 7.10 (d, J =8.4Hz, 1H), 4.62 (d, J =6....
Embodiment 2
[0053] (1) Preparation of 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine (Ⅵ):
[0054]
[0055] Add 15g of 4-chloro-5-ethoxycarbonyl-2-methylthiopyrimidine and 18ml of triethylamine to dissolve in 130ml of tetrahydrofuran respectively, cool down to 0°C, and add 13.5g of 3-chloro-4-methoxy in batches Benzylamine, react at 0-5°C for 30 minutes, then react at 20-30°C, and detect the end point of the reaction by HPLC. The reactant was concentrated under reduced pressure, and 120 ml of ethyl acetate and citric acid aqueous solution were added for washing. The organic phase was washed with water and brine, dried, filtered, and concentrated under reduced pressure to obtain 22 g of crude product. Finally, dissolve the crude product with absolute ethanol at 55-60°C, stir and crystallize overnight, filter, and dry to obtain 15 g of the product.
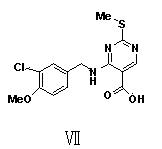
[0056] (2) Preparation of 4-(3-chloro-4-methoxybenzylamino-2-(methylthio)pyrimidine-5-carboxylic acid (VII):
...
Embodiment 3
[0069] (1) Preparation of 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine (Ⅵ):
[0070]
[0071] Add 2.32g of 4-chloro-5-ethoxycarbonyl-2-methylthiopyrimidine and 3.06ml of triethylamine to dissolve in 30ml of ethyl acetate respectively, cool down to 0~5℃, add 2.28g of 3-chloro-4 -Methoxybenzylamine, react at 0~5°C for 30min, then react at 20~30°C, and detect the end point of the reaction by HPLC. The reactant was concentrated under reduced pressure, washed by adding 120 ml each of ethyl acetate and citric acid aqueous solution, and the organic phase was washed with water and brine, dried, filtered, and concentrated under reduced pressure to obtain 3.1 g of crude product. Finally, dissolve the crude product with absolute ethanol at 55-60°C, stir and crystallize overnight, filter, and dry to obtain 2.0 g of the product.
[0072] (2) Preparation of 4-(3-chloro-4-methoxybenzylamino-2-(methylthio)pyrimidine-5-carboxylic acid (VII):
[0073]
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com