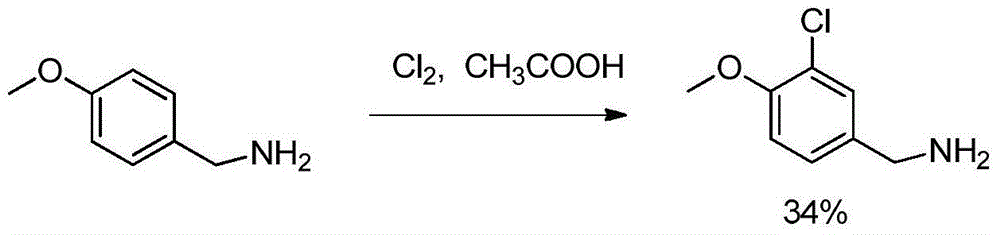
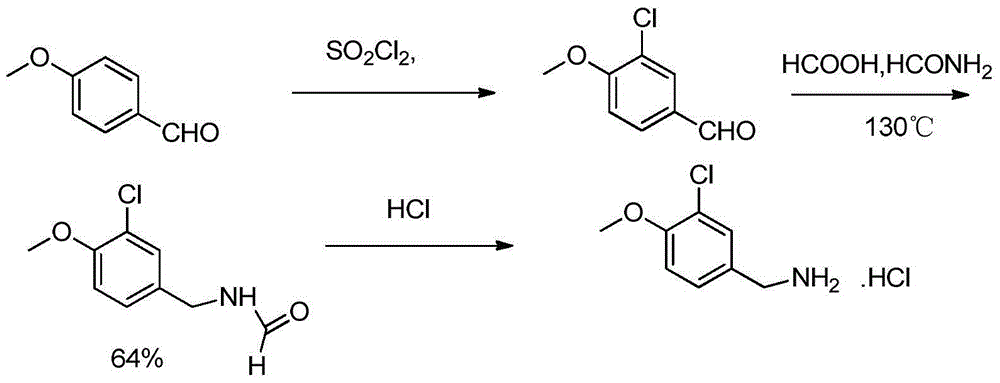
Preparation method of avanafil important intermediate
A technology of avanafil and intermediates, which is applied in the field of preparation of important intermediates of avanafil, can solve the problems of harsh reaction conditions, great health hazards for operators, and high equipment requirements, and achieve low equipment requirements and short process routes , the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
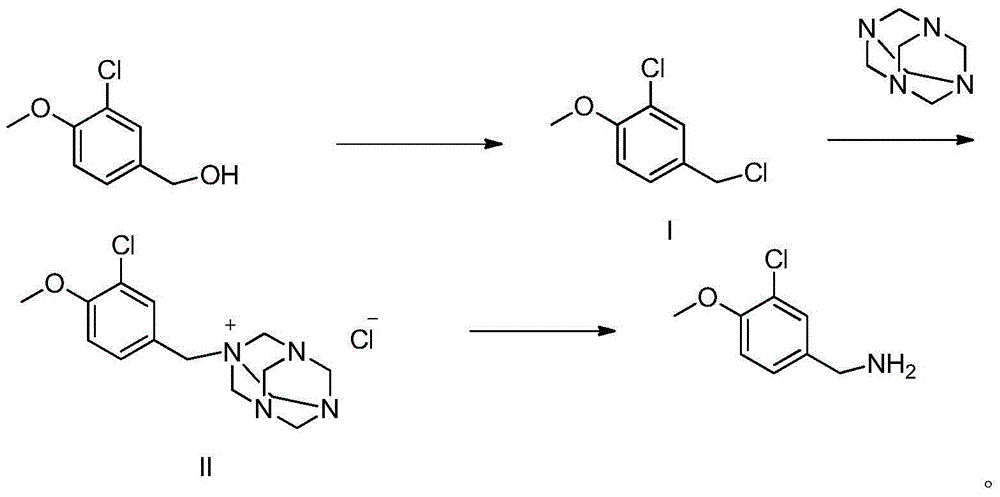
Embodiment 13
[0024] The preparation of embodiment 13-chloro-4-methoxybenzyl chloride (compound I)
[0025] Add 800ml of dichloromethane, 138g of 3-chloro-4-methoxybenzyl alcohol and 5ml of DMF into a 2L three-necked flask, and add 96g of thionyl chloride dropwise under stirring. After the dropping, the temperature was slowly raised to reflux until HPLC showed that 3-chloro-4-methoxybenzyl alcohol was less than 0.5%. After cooling down to below room temperature, 800ml of saturated sodium bicarbonate solution was slowly added, and the mixture was stirred for 10min to separate the layers. The aqueous layer was extracted with dichloromethane, the organic layers were combined, washed with water, dried over anhydrous magnesium sulfate, and rotary evaporated in a water bath at 35°C to obtain 144g of 3-chloro-4-methoxybenzyl chloride with a yield of 94.3%, [M+H] + =191.
Embodiment 2
[0026] The preparation of embodiment 2 compound II
[0027] Add 500ml of absolute ethanol and 93g of urotropine into a 1L three-necked flask, and add 114g of 3-chloro-4-methoxybenzyl chloride dropwise at a controlled temperature of 20-30°C. After dropping, the temperature was slowly raised to reflux for 2 hours, and the temperature was lowered to 20°C to obtain compound II, which was directly put into the next reaction without separation.
Embodiment 33
[0028] The preparation of embodiment 33-chloro-4-methoxybenzylamine
[0029] 350 g of concentrated hydrochloric acid was added dropwise to Example 2, and the temperature was slowly raised to 50° C. for 1 h after the drop was completed. Cool down to 20°C, filter, adjust the filtrate to pH 13 with sodium hydroxide, and rectify under reduced pressure to collect fractions at 110-115°C (1mmHg) to obtain 81g of colorless oily 3-chloro-4-methoxybenzylamine , yield 80%. [M+H] + =172, 1 HNMR (DMSO-D 6 ): 3.86 (3H, s), 3.94 (2H, s), 7.19 (1H, d), 7.31 (1H, dd), 7.61 (1H, d,), δ8.40 (2H, brs).
[0030] Embodiment 4-7 is tested according to the following parameters, and the rest refer to the corresponding steps of embodiment 1-3.
[0031]
[0032]
[0033] The compounds obtained in Examples 4-8 are identical to the 3-chloro-4-methoxybenzylamine prepared in Example 3 through structural confirmation. The total yield of 3-chloro-4-methoxybenzylamine prepared in Examples 4-8 of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com