Compound, crystal form compound and preparation method thereof
A technology of crystallization and avanafil, applied in the field of drug synthesis, can solve the problems of exceeding the limit of impurities, difficult to meet the standards of drug use, poor stability, etc., and achieve the effects of low single impurity content, good stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
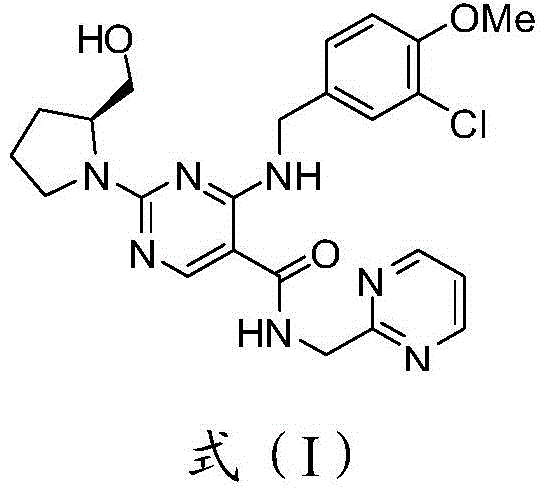
[0026] The present invention also provides a preparation method of avanafil fumarate, comprising: reacting avanafil and fumaric acid to obtain avanafil fumarate.
[0027] According to the present invention, avanafil is reacted with fumaric acid, wherein, the present invention has no special restriction on the source of said avanafil, it can be made or purchased, and there is no special requirement on the purity of avanafil, The crude product prepared by the reaction is sufficient, such as a crude product with a purity of 99.0%; the molar ratio of the avanafil to the fumaric acid is preferably 1:(1-2), more preferably 1:1; The solvent of said reaction is preferably one or more of methanol, ethanol, aqueous methanol and aqueous ethanol, more preferably one or more of methanol and ethanol; the temperature of said reaction is preferably 20 to 70°C, more preferably Preferably it is 50 to 68°C.
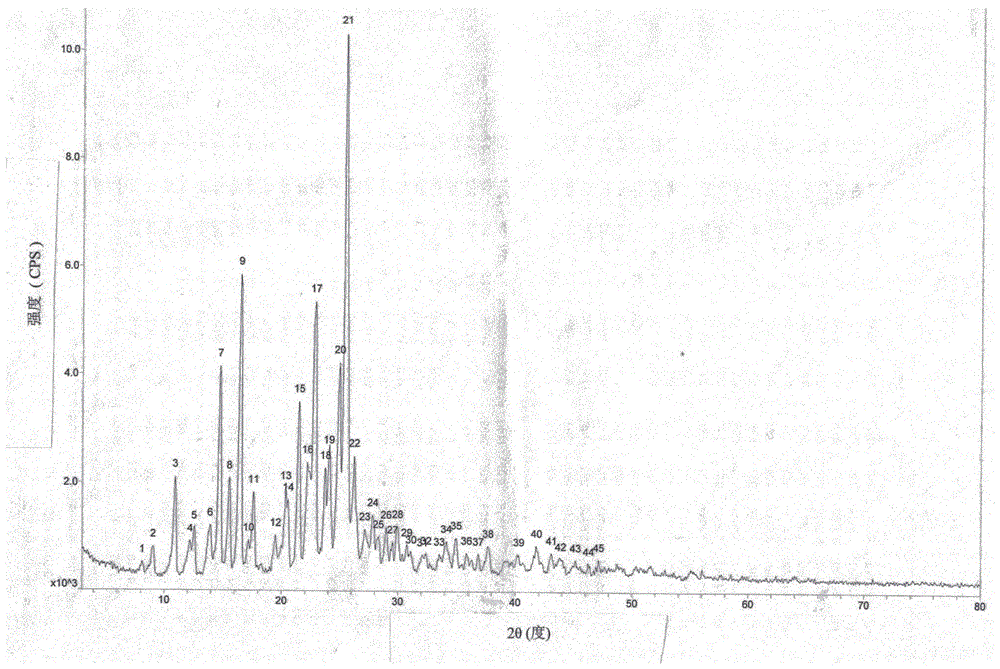
[0028] The present invention also provides avanafil fumarate crystals, which are chara...
Embodiment 1
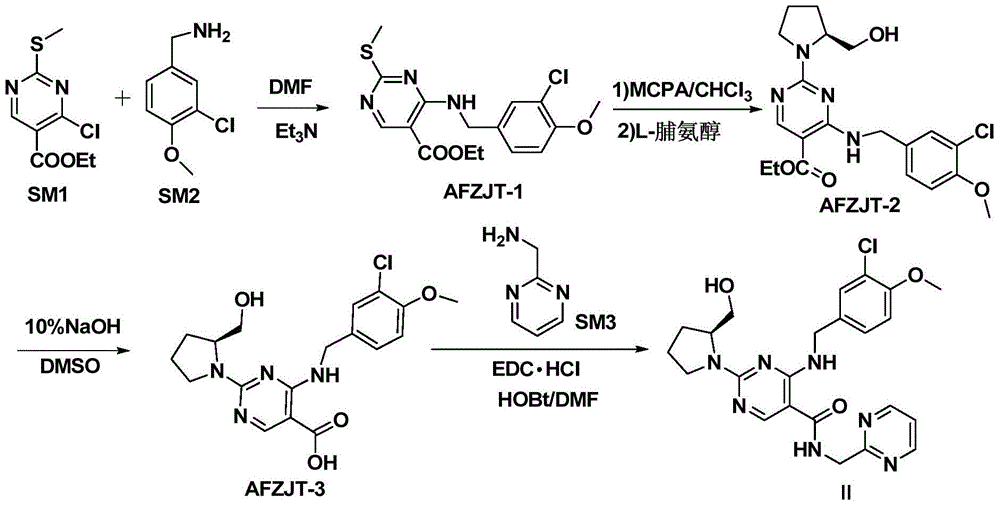
[0040] In a 1L three-necked flask, add (39.28g, 0.10mol) (S)-4-(3-chloro-4-methoxybenzylamino)-5-carboxylic acid-2-(2-hydroxymethyl-1 -pyrrolidinyl)pyrimidine (AFZJT-3), (21.84g, 0.15mol) 2-aminomethylpyrimidine hydrochloride, (28.76g, 0.15mmol) 1-(3-dimethylaminopropyl)-3 -Ethylcarbodiimide hydrochloride, (20.26g, 0.15mol) 1-hydroxybenzotriazole, 0.8L N,N-dimethylformamide, (30.36g, 0.30mol) triethylamine, 30 React at ℃ for 24 hours, HPLC shows that the reaction is complete, add 1.2L of water, extract with dichloromethane, wash twice with about 5% sodium carbonate aqueous solution, wash once with deionized water, dry over anhydrous sodium sulfate, filter with suction, remove the dryness agent, dichloromethane solvent was concentrated under reduced pressure, ethyl acetate was added to make a slurry, suction filtered, and dried under reduced pressure at 50°C for 5 hours to obtain 40.72g of avanafil-enriched crude product with a yield of 84.13%.
[0041] 10 g of the crude produ...
Embodiment 2
[0044] At room temperature, in a 500ml three-neck flask, add methanol 200.0mL, the crude product of avanafil (9.68g, 20.0mmol) prepared in Example 1, fumaric acid (2.32g, 20.0mmol), under stirring, heat up to reflux, dissolve After clearing, the heating was stopped, and the stirring was continued for 4 hours to precipitate a solid, and dried under reduced pressure at 50° C. for 5 hours to obtain 10.94 g of avanafil fumarate crystals, with a yield of 91.17%.
[0045] The avanafil fumarate prepared in the example was detected by HPLC, and the results showed that its purity was 99.84%, the largest single impurity was 0.045%, and other relatively large impurities were 0.039%.
[0046] The avanafil fumarate prepared in the example was placed at 60°C and light for 10 days, and its stability was detected by HPLC. The results are shown in Table 1, which shows the avanafil fumarate provided in the examples of the present invention. Stability results of nafil fumarate and avanafil at 60...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com