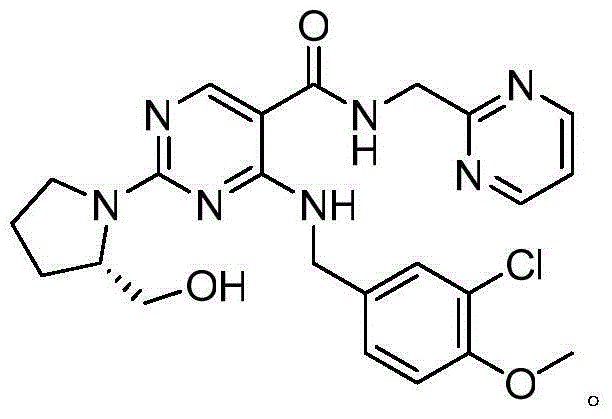
Avanafil intermediate as well as preparation method and application thereof
A technology for avanafil and intermediates, which is applied in the field of medicinal chemistry, can solve the problems of nitrogen oxide residues, unsuitable for industrialization, etc., and achieves the effects of easy products, reduced preparation costs, and easy availability of reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
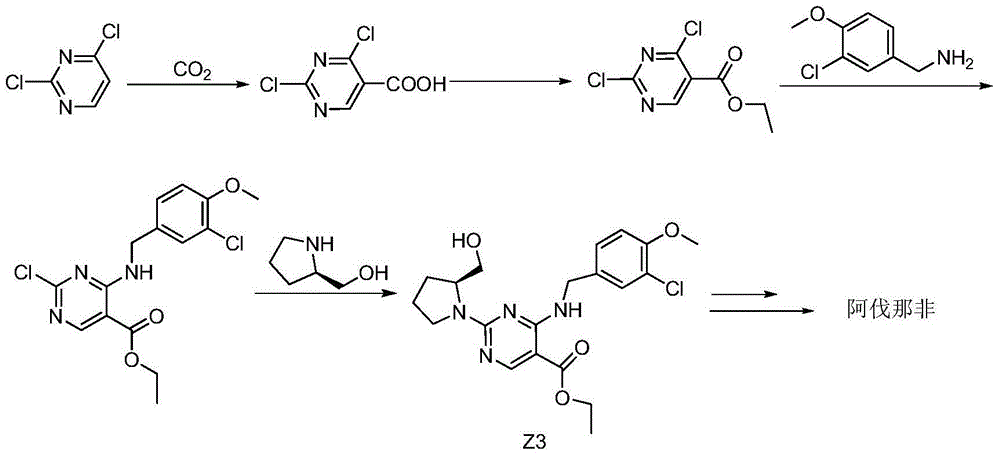
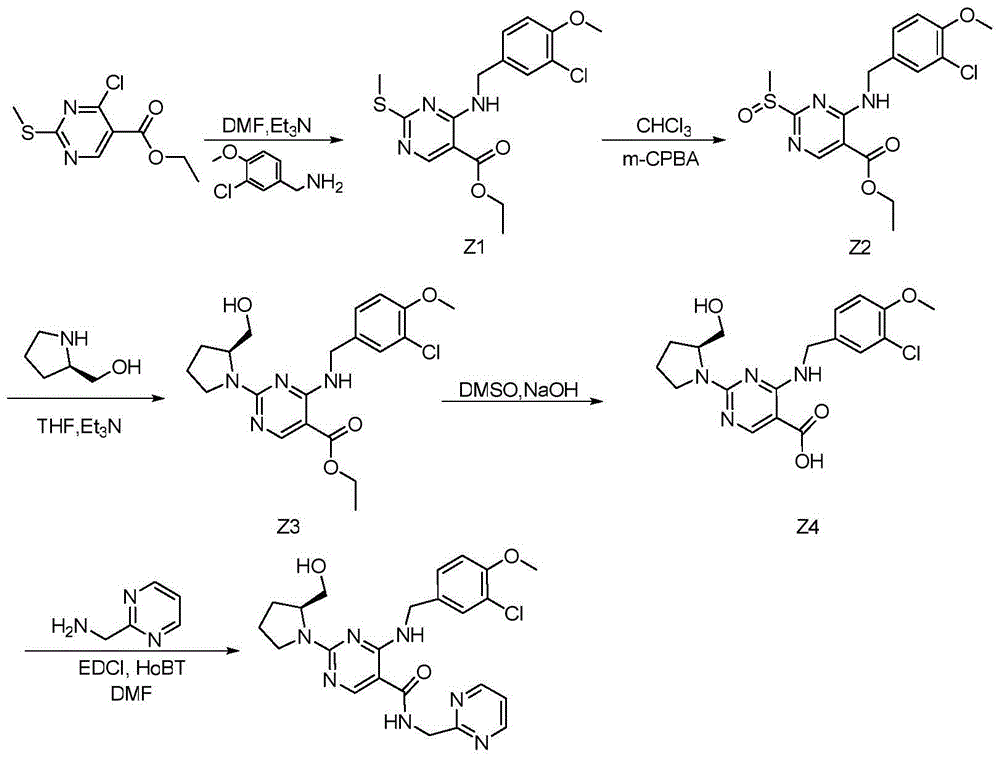
[0038] Embodiment 1: prepare the intermediate of the present invention
[0039] Step a: prepare the compound shown in formula II
[0040]
[0041] Add 12.9g proline methyl ester hydrochloride (compound shown in formula I), 17.2g oxymethylisourea sulfate and 65mL methanol in the 250mL there-necked flask that is equipped with reflux condenser, thermometer; Heat to reflux; Reflux After reacting for 16 hours, the reaction was terminated, and the temperature was lowered to 0° C. to prepare a methanol solution containing the compound represented by formula II for future use.
[0042] Step b: prepare the compound shown in formula III
[0043]
[0044] Add 18.9g sodium methoxide and 30mL methanol to the methanol solution containing the compound shown in formula II prepared in step a;
[0045] Control the reaction temperature below 10°C, add 21.6g of ethoxymethylene dropwise; after the dropwise addition, return to room temperature and react for 16 hours; after the reaction is c...
Embodiment 2
[0068] Embodiment 2: Application of the intermediate of the present invention to prepare avanafil
[0069] Step ①: prepare the compound shown in formula V
[0070]
[0071] Add 26.9g of the intermediate of the present invention prepared in Example 1 and 150mL of methanol to a 250mL three-necked flask equipped with a reflux condenser and a thermometer; 2.3g of sodium borohydride was added; after the addition, the temperature was slowly raised to room temperature and reacted for 3 hours; after the reaction was completed, the pH of the system was adjusted to neutrality with concentrated hydrochloric acid; 25 g of the compound represented by formula V was obtained as a white solid with a molar yield of 90% and an HPLC purity of 98%.
[0072] Step ②: prepare the compound shown in formula VI
[0073]
[0074] Add 25g of the compound shown in formula V and 100mL dimethyl sulfoxide to a 250mL three-necked flask equipped with a reflux condenser and a thermometer, and stir until...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com