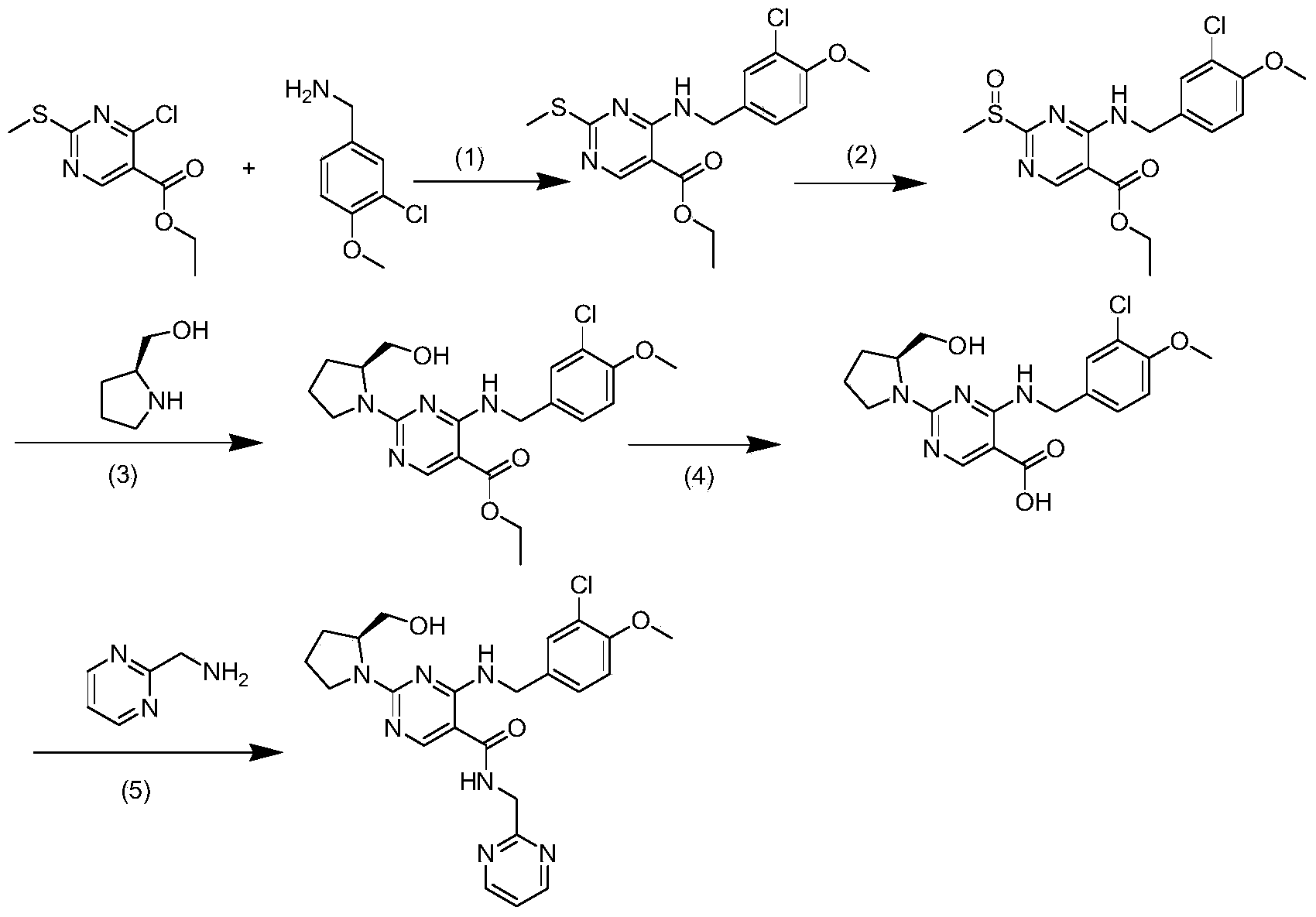
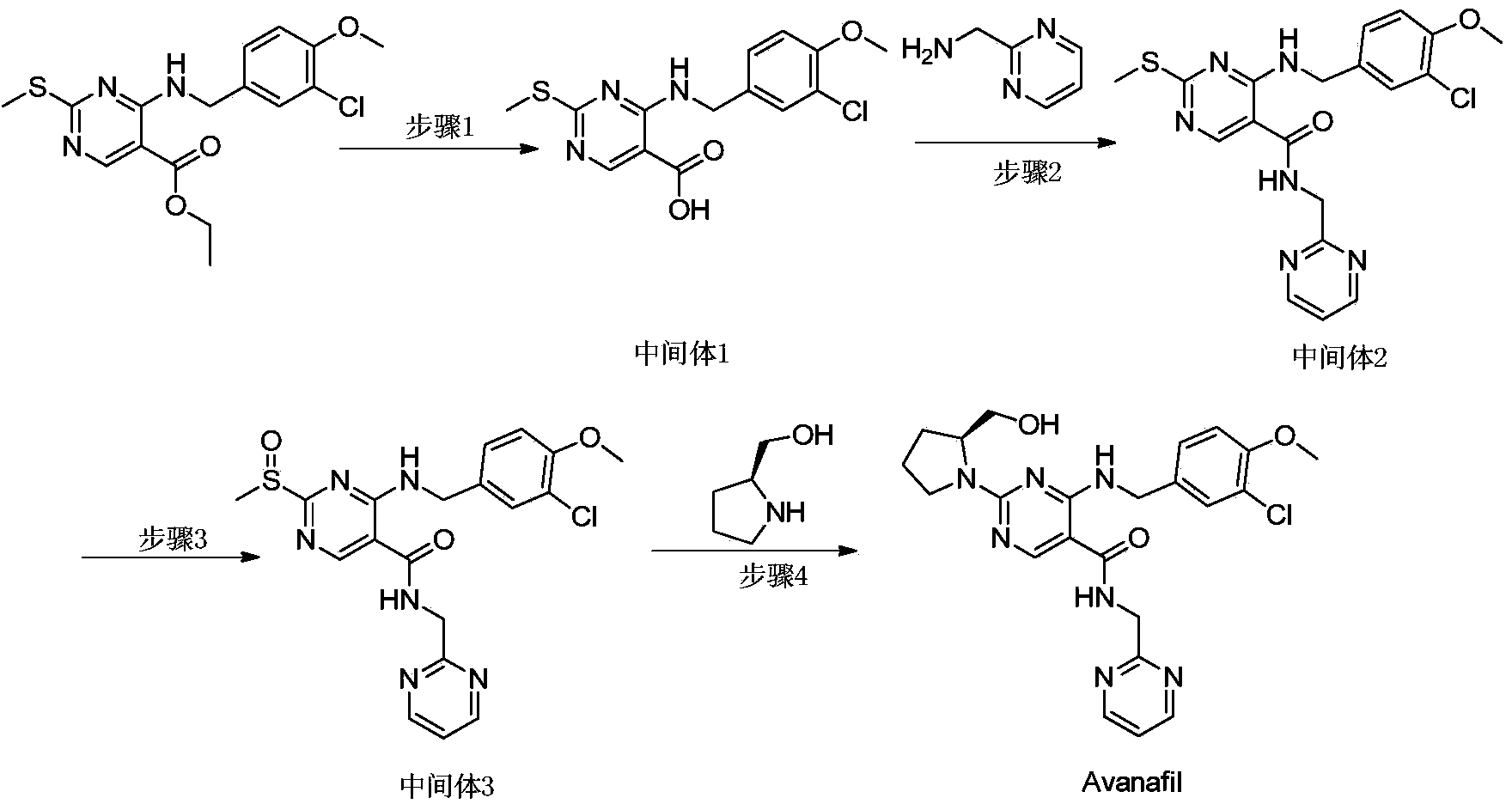
Method for preparing avanafil
A technology of avanafil and methoxybenzylamino, which is applied in the field of compound preparation, can solve the problems of yield and manufacturing cost (high cost, difficult to guarantee product quality, difficult curing and purification, etc.), and achieve low cost and easy operation The effect of simplicity, ease of purification and detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of Avanafil
[0043] The preparation method of avanafil, comprises the steps:
[0044] 1) Preparation of Intermediate 1: Dissolve 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine (5g) in ethanol (15ml), Add purified water (5ml) and sodium hydroxide (1.1g), reflux and stir for 1 hour; after TLC monitors that the raw materials disappear, concentrate the ethanol to dryness under reduced pressure, adjust the pH to 6-7 with concentrated hydrochloric acid, precipitate the solid, and collect the solid by suction filtration After drying, Intermediate 1 (4.2g) was obtained with a yield of 90%;
[0045] 2) Preparation of intermediate 2: at room temperature, 4-(3-chloro-4-methoxybenzylamino)-5-carboxy-2-methylthiopyrimidine (4.0g), 2-aminomethylpyrimidine (2.2g), EDCI (2.8g) and N-hydroxybenzotriazole (1.9g) were added to DMF (40 mL), stirred for 10 hours; after TLC detected that the reaction was complete, the reaction s...
Embodiment 2
[0049] Example 2 Preparation of Avanafil
[0050] The preparation method of avanafil, comprises the steps:
[0051] 1) Preparation of Intermediate 1: Dissolve 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine (4g) in ethanol (12ml), Purified water (4 ml) and potassium hydroxide (1.2 g) were added thereto, followed by stirring at 40°C for 10 hours. After the disappearance of the raw material was monitored by TLC, the ethanol was concentrated under reduced pressure to dryness, and the pH was adjusted to 6-7 with concentrated hydrochloric acid. After the solid was precipitated, it was filtered with suction, and the solid was collected and dried to obtain Intermediate 1 (3.4 g), with a yield of 92%;
[0052] 2) Preparation of Intermediate 2: Add 4-(3-chloro-4-methoxybenzylamino)-5-carboxy-2-methylthiopyrimidine (4.0 g) to thionyl chloride ( 30mL), reflux for 2 hours, concentrate to dryness under reduced pressure, add dichloromethane to the resi...
Embodiment 3
[0055] Example 3 Preparation of Avanafil
[0056] The preparation method of avanafil, comprises the steps:
[0057] 1) Preparation of Intermediate 1: Dissolve 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine (5g) in ethanol (15ml), Purified water (5 ml) and lithium hydroxide (0.6 g) were added, and the mixture was stirred under reflux for 4 hours. After the disappearance of the raw material was monitored by TLC, the ethanol was concentrated under reduced pressure to dryness, and the pH was adjusted to 6-7 with concentrated hydrochloric acid. After the solid was precipitated, it was filtered with suction, and the solid was collected and dried to obtain Intermediate 1 (4.3 g), with a yield of 92%;
[0058] 2) Preparation of intermediate 2: 4-(3-chloro-4-methoxybenzylamino)-5-carboxy-2-methylthiopyrimidine (2.0 g) in dichloromethane (20 mL) at 0 °C ) solution was added triethylamine (0.9g) and ethyl chloroformate (0.96g), stirred at room tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com