Method for detecting avanafil and related impurities thereof
A detection method and avanafil technology, which can be used in measurement devices, instruments, scientific instruments, etc., can solve problems such as identification of unimpurity structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
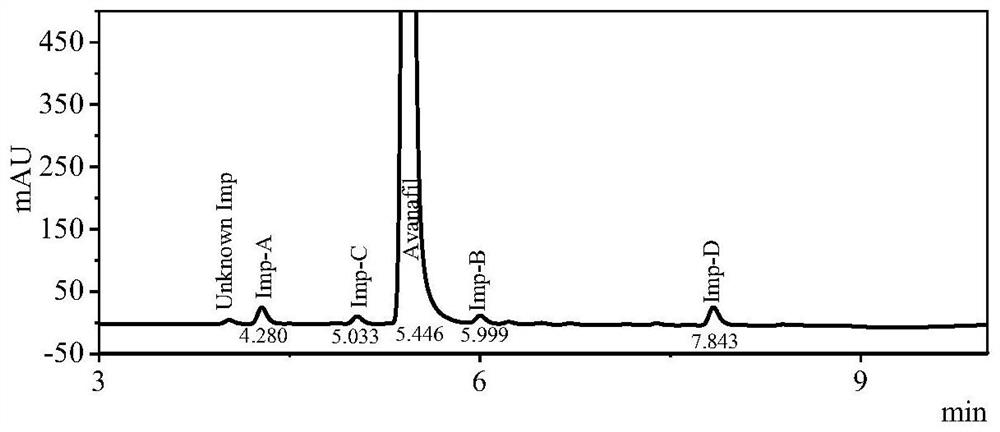
[0023] Avanafil test solution 1 is detected by UPLC method, and the results are as follows: figure 1 Shown, the content of impurity A, B, C, D is respectively 0.89%, 0.57%, 0.57% and 1.44%.
[0024] Avanafil test solution 1 was separated and identified by LC-MS method. The results showed that there was a [M+H]+ peak of 393.2 in impurity A, a [M+H]+ peak of 430.8 in impurity B, and a [M+H]+ peak of 450 in impurity C. The [M+H]+ peak and the impurity D have a [M+H]+ peak of 858.0.
[0025] Table 1 and Table 2 are the detection data of avanafil test solution 1.
[0026] Table 1 Retention time and resolution of avanafil and its related impurities
[0027] name retention time (min) Separation Impurity A 4.29 - Impurity C 5.02 5.35±0.03 Avanafil 5.44 2.81±0.03 Impurity B 6.02 3.88±0.03 Impurity D 7.85 12.35±0.1
[0028] Table 2 Linearity and Sensitivity
[0029]
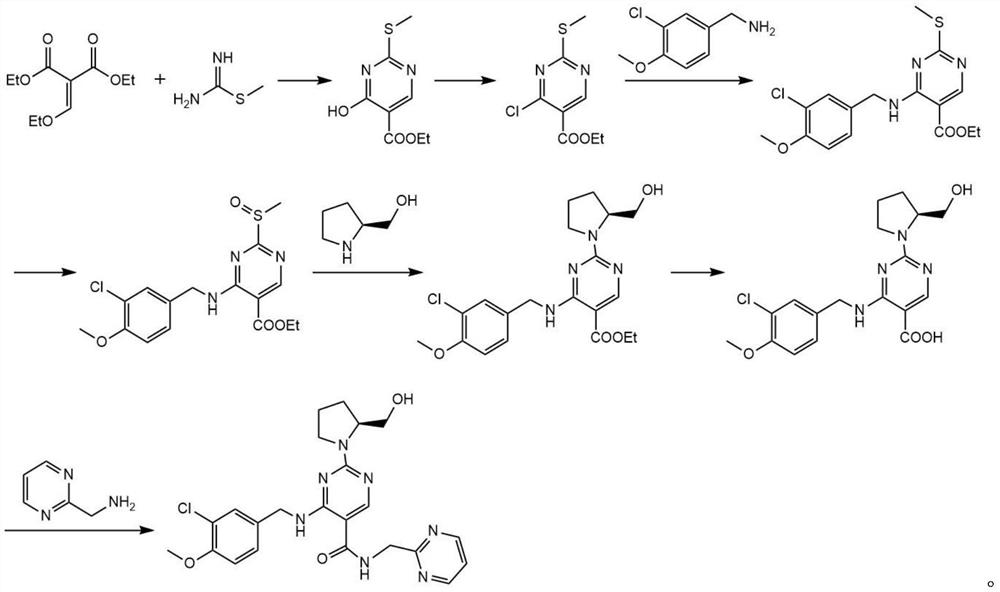
[0030] After detection and verification, impurity A is 4-{[(...
Embodiment 2
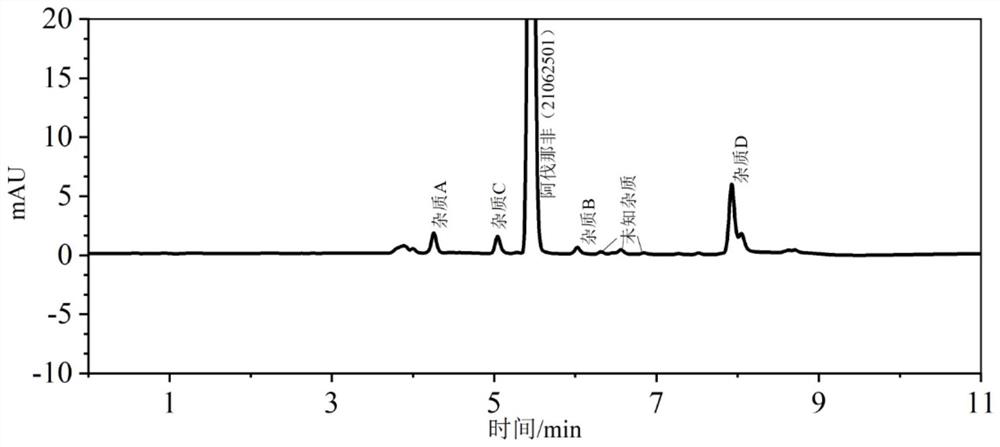
[0032] Avanafil test solution 2 is detected by UPLC method, and the results are shown in Table 3:
[0033] Table 3 Content and resolution of avanafil and related impurities
[0034]
[0035]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com