Spiral donor organic light-emitting small molecule material containing alkyl sulfur atom and its preparation method and application
A technology of alkyl sulfide and small molecules, which is applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of complex preparation process and single light color, and achieve good bipolar transmission characteristics and high fluorescence quantum production rate, the effect of high horizontal orientation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
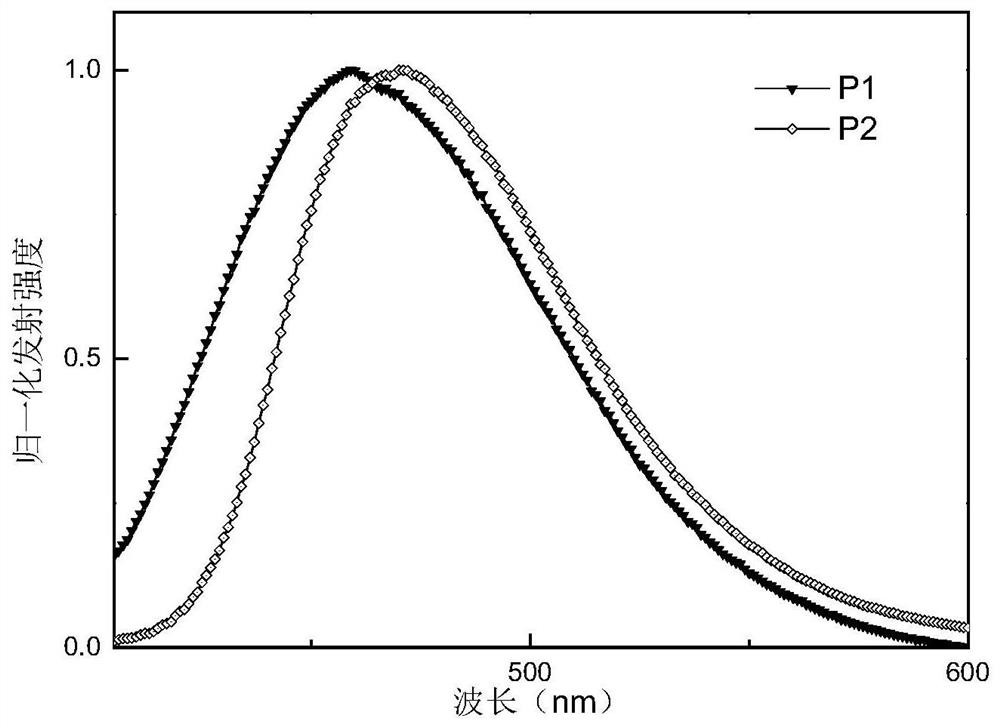
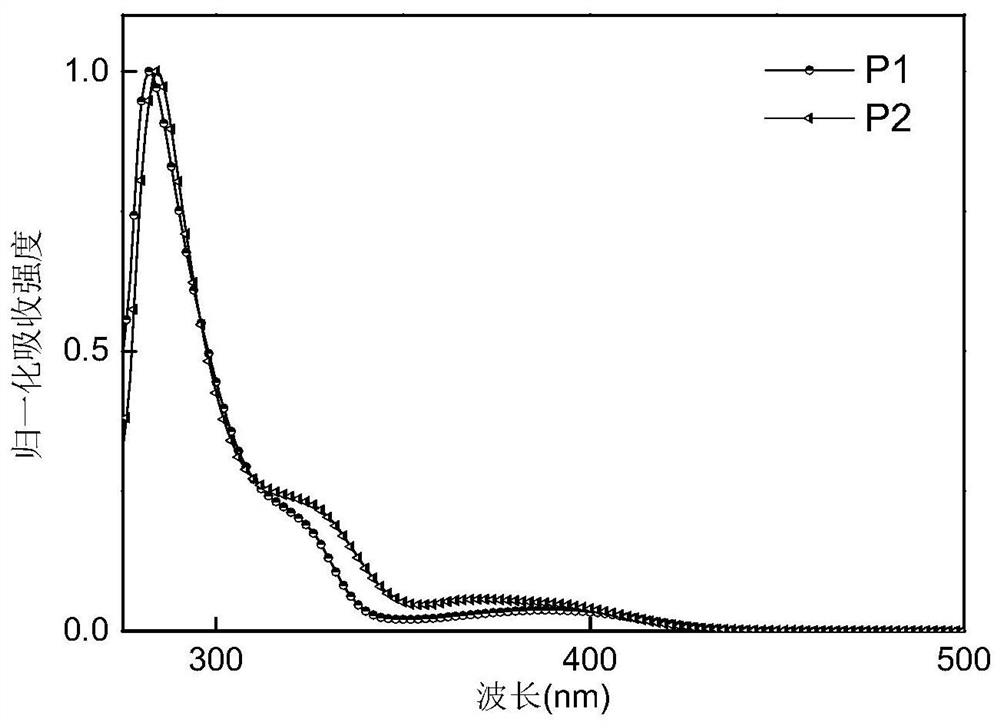
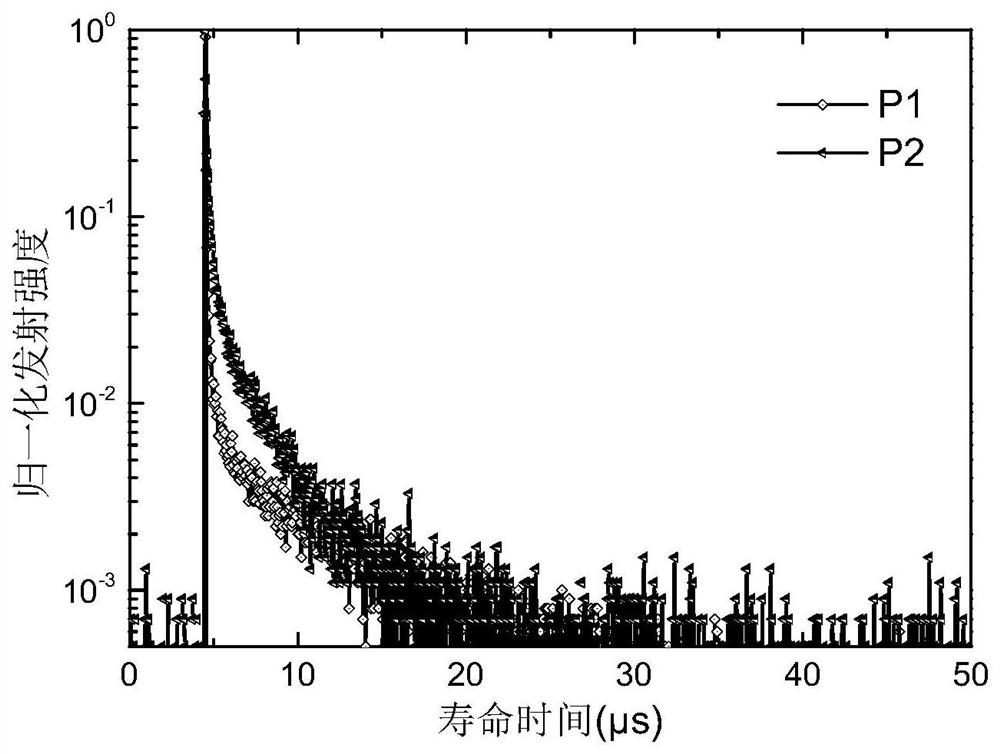
[0051] This embodiment prepares intermediates 1 to 10 and compound P1:
[0052] The preparation steps of intermediate 1 (2-iodine 10H-spiro[acridine-9,9'-thioxanthene]) are as follows:
[0053]
[0054] Under a nitrogen atmosphere, 1.0 g (2.8 mmol) of spiro[acridine-9,9'-thioxanthene] and 0.63 g (2.8 mmol) of N-iodosuccinimide (NIS) were successively added to a 250 ml three-necked flask. , dichloromethane (DCM) 80 mL. Heated to 50°C and stirred for 24 hours in the dark. After the reaction, the solvent in the reaction system was suspended to dryness, extracted three times with dichloromethane and water, and the organic phase was taken. Dichloromethane was distilled off under reduced pressure, and purified by a silica gel column to obtain 0.95 g of the intermediate of structural formula 1, with a yield of 90%, C25H16INS, M / Z=489.37. m / z: 489.00 (100.0%), 490.01 (27.0%), 491.00 (4.5%), 491.01 (2.7%), 492.00 (1.2%); elemental analysis: C, 61.36; H, 3.30; I, 25.93; N , 2.86;...
Embodiment 2
[0087] Compound P2 (10-(3-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-10H-spiro[acridine-9,9'- thioxanthene]), its structural formula and synthetic route are as follows:
[0088]
[0089] The specific implementation steps are:
[0090] Under a nitrogen atmosphere, 1.0 g (2.8 mmol) of 10H-spiro[acridine-9,9'-thioxanthene], 2-(3-bromophenyl)-4,6-diphenyl 1.1 g (2.8 mmol) of 1,3,5-triazine, 0.90 g sodium tert-butyl alkoxide, 59.8 mg palladium acetate and tri-tert-butylphosphine were added, heated to 110°C, and stirred for 24 hours in the dark. After the reaction, the solvent in the reaction system was suspended to dryness, extracted three times with dichloromethane and water, and the organic phase was taken. Dichloromethane was distilled off under reduced pressure, and purified by a silica gel column to obtain 1.45 g of the product of structural formula P2, with a yield of 80%. Molecular formula: C46H30N4S; M / Z=670.83 Theoretical value: m / z: 670.22 (100.0%), 671.22 (49.8%), 67...
Embodiment 3
[0092] This example prepares compound P3 (2-(9H-carbazol-9-yl)-10-(4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl) -10H-spiro[acridine 9,9'-thia]), its structural formula and synthetic route are as follows:
[0093]
[0094] The specific implementation steps are:
[0095] Under a nitrogen atmosphere, 1.0 g (2.4 mmol) of the intermediate 3,2-(4-bromophenyl)-4,6-diphenyl-1,3,5-triazine 1.1 g was successively added to a 250 ml three-necked flask (2.8mmol), 0.90g of sodium tert-butyl alkoxide, and then add 59.8mg of palladium acetate and tri-tert-butylphosphine, heat to 110°C, and stir for 24 hours in the dark. After the reaction, the solvent in the reaction system was suspended to dryness, extracted three times with dichloromethane and water, and the organic phase was taken. Dichloromethane was distilled off under reduced pressure, and purified by a silica gel column to obtain 1.20 g of the product of structural formula P3, with a yield of 76%. Molecular formula: C58H37N5S; M / Z=...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com