A kind of oligomer based on fluorene and its synthesis method and application
An oligomer and phenyl technology, which is applied in the field of new fluorene-based oligomers and their synthesis, can solve the problem of high work function, and achieve the effects of lowering potential barriers, single structure, good solubility and film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] 1) Synthesis of 2-bromo-9,9-bis(2-ethylmorpholine)fluorene (M1):
[0037] Dissolve 2-bromofluorene (4.9g, 20mmol), 4-(2-chloroethyl)morpholine hydrochloride (9.3g, 50mmol) and potassium hydroxide (11.2g, 200mmol) in 200mL of tetrahydrofuran. The reaction was conducted at 80°C for 48h under water and oxygen-free Ar environment. After the reaction, the solvent tetrahydrofuran was distilled under reduced pressure. The product was first purified by column chromatography (silica gel, ethyl acetate: methanol = 10:1 as the eluent) to obtain a milky white solid recrystallized from petroleum ether, and finally white crystals 6.5 g, the yield was 69%.
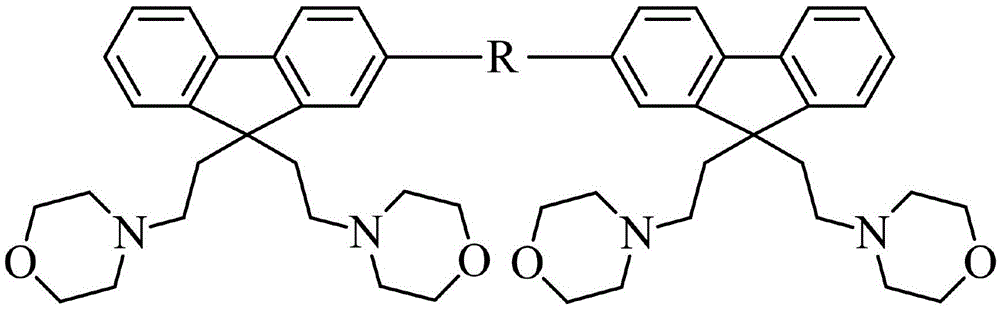
[0038] 2) Synthesis of 1,4-bis[9,9-bis(2-ethylmorpholine)fluorene]benzene (FNO-B-FNO):
[0039] In a 50mL three-necked flask, M1 (471mg, 1mmol), 1.4-benzenediboronic acid (83mg, 0.5mmol), palladium tetraphenylphosphide (15mg, 0.01mmol), potassium carbonate (1.38g, 10mmol) were added to 10mL Toluene 5mL water mixture. The reaction sys...
example 2
[0041] 1) Synthesis of 2,7-dibromo-9,9-dioctylfluorene (M2)
[0042] Put 2,7-dibromofluorene (5g, 15.5mmol), tetrabutylammonium bromide (0.1g, 0.31mmol), 200mL dimethyl sulfoxide in a three-necked flask under vigorous stirring to form a suspension, and drop in 50wt % Sodium hydroxide aqueous solution 5mL, after 1h reaction, then 1-bromooctane (6.2g, 31mmol) was added dropwise. The reaction mixture was stirred at room temperature for 6 hours, then the reaction was stopped, and extracted with ether (150 mL×3), the organic phase was washed with saturated brine, and dried over anhydrous magnesium sulfate. The solvent was distilled under reduced pressure, and the product was recrystallized in a methanol / acetone mixed solvent to obtain 8 g of white needle crystals, with a yield of 80%.
[0043] 2) Synthesis of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborane-diyl)-9,9-dioctylfluorene (M3)
[0044] Dissolve M2 (5.4g, 10mmol) in 60mL tetrahydrofuran, add 15mL 1.6mol / L n-butyllithium dropwise ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com