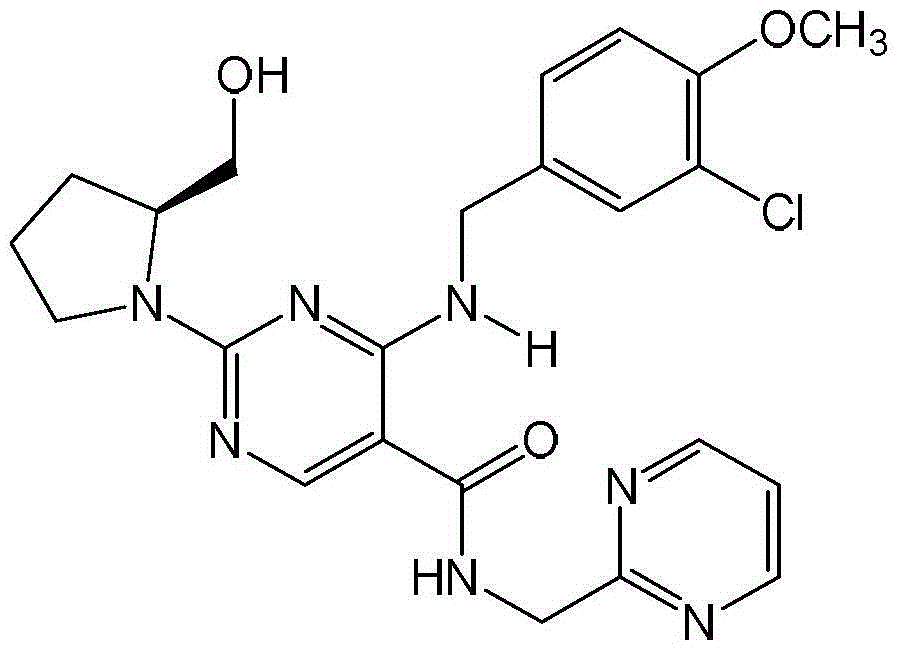
Amorphous form of avanafil, preparation method of, application and medicine composition of amorphous form of avanafil
A kind of avanafil and amorphous technology, applied in the field of chemical pharmaceuticals, can solve the problems of reduced probability of interaction, short half-life, etc., and achieve the effect of mild conditions, stable drug properties and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Amorphous
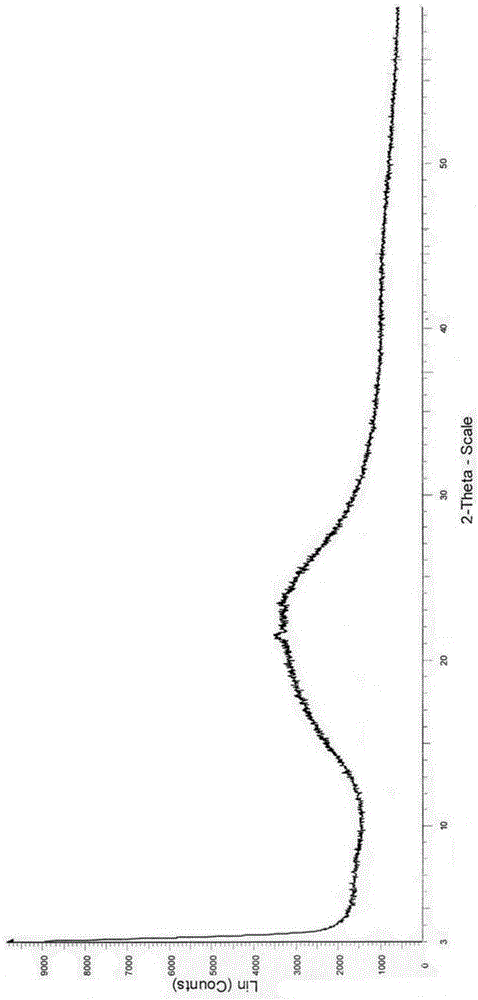
[0025] Add 0.5 g of crude avanafil and 10 ml of ethyl acetate into the reaction flask, stir at room temperature until the avanafil dissolves, keep for 30 minutes, and filter. The filtrate was concentrated to dryness under reduced pressure to obtain a white solid, which was then dried under reduced pressure at 60°C to constant weight to obtain the amorphous avanafil of the present invention, m.p.76.0-77.5°C. The measured content is 98.98%.
[0026] Use the Rigaku Dmax / 2400 type X-ray polycrystalline powder diffractometer (condition: Cu Kα, 40kV, 100mA) to measure and get figure 1 Powder X-ray Diffraction Spectrum shown.
experiment example 1
[0031] Comparing the amorphous drug prepared in Example 1 with the crystalline drug prepared in the comparative example, and administering it orally to rats, the amorphous drug only needs 80% of the dosage of the crystalline drug to achieve the same therapeutic effect.
experiment example 2
[0033] The subjects took a blood sample in the morning, and then took 500mg of avanafil amorphous form, crystalline form and 150ml of water respectively on an empty stomach, and took blood samples at 0.25, 0.5, 1, 1.5, 2, 3, 4 and 6 hours after taking the medicine. Serum was separated and stored at -20°C for future use. Microbial detection technology was used to measure drug concentration data in serum at different times. The experimental results show that the time for the blood concentration of avanafil amorphous form to reach the peak is 35 minutes, and the time for the blood concentration of avanafil amorphous form of the present invention to reach the peak value is 30 minutes. Compared with crystalline drugs, amorphous drugs have faster absorption and better bioavailability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com