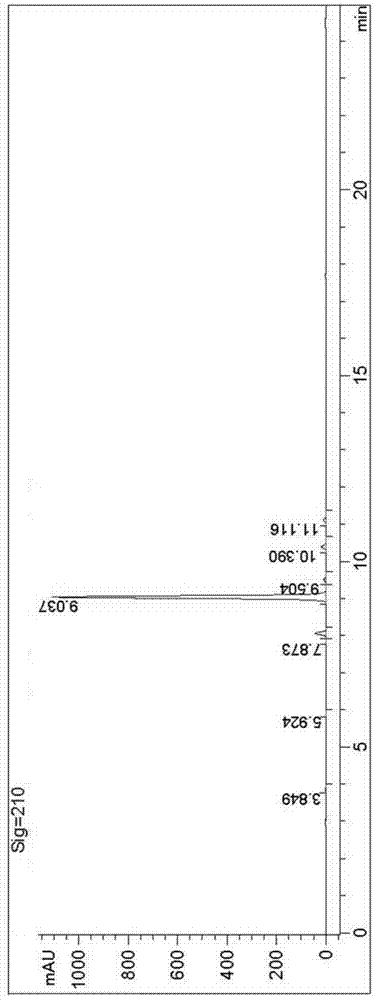
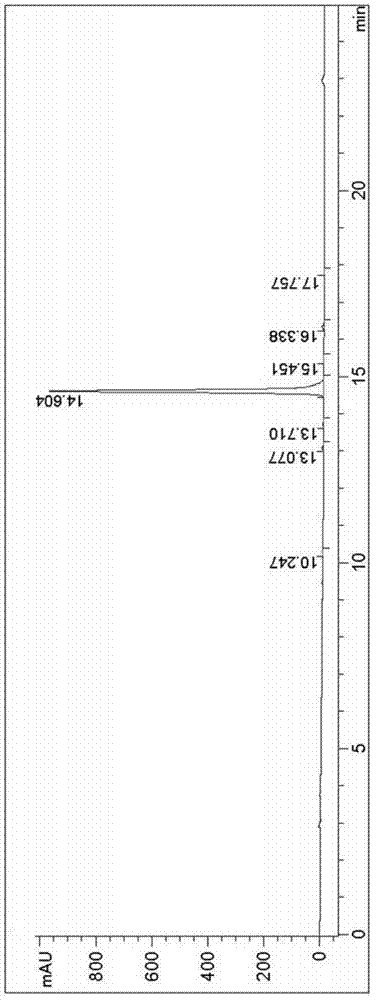
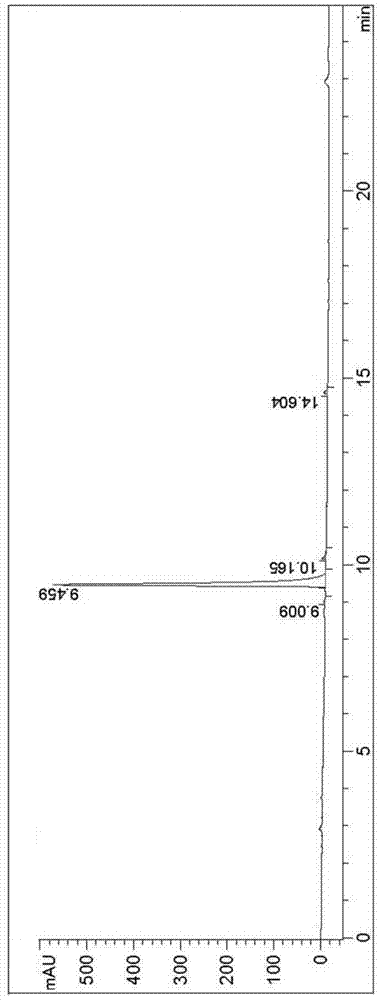
Avanafil
A technology of ivanafil and intermediates, applied in the field of drug synthesis, can solve the problems of affecting product yield, high synthesis cost, long synthesis time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] At room temperature, 21.1g of 2,4-dichloro-5-pyrimidinecarbonyl chloride was added to the reaction flask and dissolved in 180mL of dichloromethane to form the first reaction solution, and 11.4g of 2-methylaminopyrimidine and 10.6g of triethylamine were dissolved in Form the second reaction solution in 100mL of dichloromethane, lower the temperature of the first reaction solution to -10°C, slowly drop the second reaction solution into the first reaction solution under this temperature condition, and slowly raise the temperature to At room temperature, add 300 mL of water, separate layers, wash the organic phase with 100 mL of water three times, dry with anhydrous sodium sulfate, filter with suction, and evaporate the organic phase to dryness under reduced pressure to obtain 25.5 g of aivanafil intermediate A2,4-dichloro -N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide.
[0070] Dissolve 25.5g of the above-prepared avanafil intermediate A2,4-dichloro-N-(2-pyrimidinylmethy...
Embodiment 2
[0073]At room temperature, 23.5g of 2,4-dimethylthio-5-pyrimidinecarbonyl chloride was added to the reaction bottle and dissolved in 200mL of chloroform to form the first reaction solution, and 11.46g of 2-methylaminopyrimidine and 7.14g of imidazole were dissolved in Form the second reaction solution in 100mL of chloroform, cool the first reaction solution to -8°C, slowly add the second reaction solution dropwise to the first reaction solution at this temperature, and slowly raise the temperature to At room temperature, add 300 mL of water, separate layers, wash the organic phase with 100 mL of water three times, dry with anhydrous sodium sulfate, filter with suction, and evaporate the organic phase to dryness under reduced pressure to obtain 28.3 g of aivanafil intermediate A2,4-dimethyl Thio-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide.
[0074] Dissolve 28.3g of the avanafil intermediate A2,4-dimethylthio-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide prepared above in...
Embodiment 3
[0077] At room temperature, 26.8g of 4-bromo-2-methylthio-5-pyrimidinecarbonyl chloride was added to the reaction bottle and dissolved in 200mL of 1,2-dichloroethane to form the first reaction solution, and 11.46g of 2-methylaminopyrimidine Dissolve 8.3g of pyridine in 100mL of 1,2-dichloroethane to form the second reaction solution, lower the temperature of the first reaction solution to -3°C, and slowly add the second reaction solution to the first reaction solution dropwise at this temperature After the dropwise addition, slowly warm up to room temperature, add 300mL of water, separate layers, wash the organic phase with 120mL of water three times, dry with anhydrous sodium sulfate, filter with suction, and evaporate the organic phase to dryness under reduced pressure to obtain 30.3g of Ava Nafil Intermediate A4-Bromo-2-methylthio-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide.
[0078] Dissolve 30.3g of Aivanafil intermediate A4-bromo-2-methylthio-N-(2-pyrimidinylmethyl)-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com