Preparation method of avanafil
A technology of avanafil and reaction solution, applied in organic chemistry methods, organic chemistry, etc., to achieve the effects of mild reaction conditions, easy operation, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
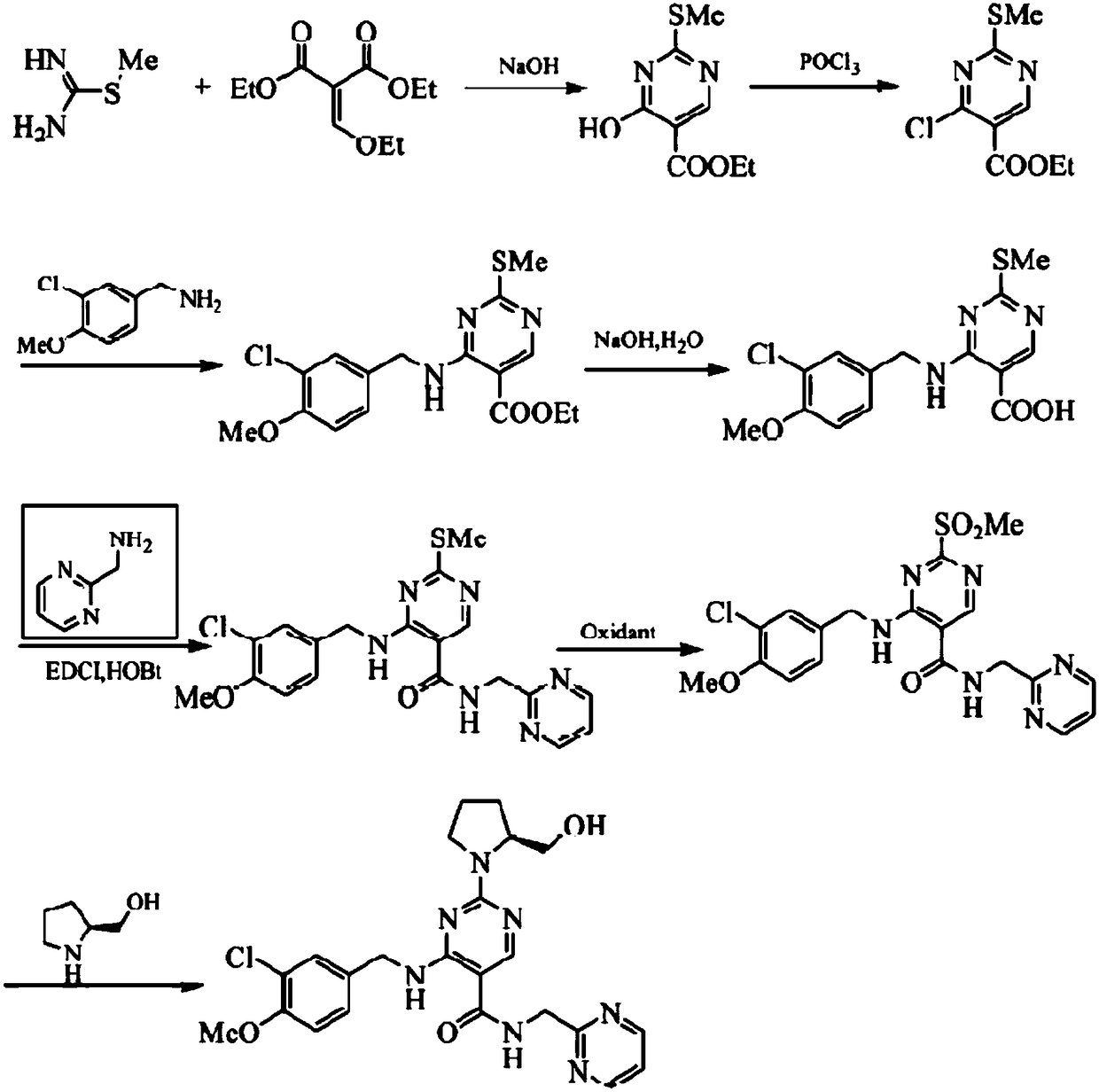
[0028] A preparation method of avanafil, comprising the steps of:
[0029] S1, adding methylthiourea sulfate (75.2g, 0.4mol) into a mass fraction of 16% NaOH (250mL, 1.18mol) solution and stirring for 30min, then diethyl ethoxymethylene malonate (103.4 g, 0.48mol) was dissolved in 160mL ethanol and slowly dripped into the reaction solution. After the dropwise addition was completed, it was reacted at room temperature for 10 hours. 99.7%); ESE-MS (m / z): 249 [M+H] + , 236.9[M+Na] + , by comparison with the literature, it can be known that it is ethyl 4-hydroxy-2-methylthiopyrimidine-5-carboxylate;
[0030] S2. Add ethyl 4-hydroxy-2-methylthiopyrimidine-5-carboxylate (55.6 g, 0.26 mol) obtained in step S1 to 150 mL of acetonitrile, stir for 25 min, and slowly add 135 mL of POCl dropwise to the reaction solution 3 After the dropwise addition is completed, the reaction solution is heated to reflux for 6 hours, then the reaction solution is cooled slightly, the reaction solution ...
Embodiment 2
[0037] A preparation process of avanafil, comprising the steps of:
[0038] S1, adding methylthiourea sulfate (75.2g, 0.4mol) into a mass fraction of 24% NaOH (155mL, 1.17mol) solution and stirring for 30min, then diethyl ethoxymethylenemalonate (103.4 g, 0.48mol) was dissolved in 160mL ethanol and slowly dripped into the reaction solution. After the dropwise addition was completed, it was reacted at room temperature for 10h, and a white solid was precipitated. Suction filtration, washing with water, and vacuum drying gave a white solid (80.5g, 94.7%) ;ESE-MS(m / z): 249[M+H] + , 236.9[M+Na] + , by comparison with the literature, it can be known that it is ethyl 4-hydroxy-2-methylthiopyrimidine-5-carboxylate;
[0039] S2. Add ethyl 4-hydroxy-2-methylthiopyrimidine-5-carboxylate (55.6 g, 0.26 mol) obtained in step S1 to 150 mL of acetonitrile, stir for 25 min, and slowly add 135 mL of PCl dropwise to the reaction solution 3 , after the dropwise addition, the reaction solution ...
Embodiment 3
[0046] A preparation process of avanafil, comprising the steps of:
[0047] S1, adding methylthiourea sulfate (75.2g, 0.4mol) into a mass fraction of 16% NaOH (250mL, 1.18mol) solution and stirring for 30min, then diethyl ethoxymethylene malonate (103.4 , 0.48mol) was dissolved in 160mL ethanol and slowly dripped into the reaction solution. After the titration was completed, it was reacted at room temperature for 7h, and a white solid was precipitated, filtered by suction, washed with water, and dried in vacuo to obtain a white solid (72.8g, 85.7%); ESE -MS(m / z): 249[M+H] + , 236.9[M+Na] + , by comparison with the literature, it can be known that it is ethyl 4-hydroxy-2-methylthiopyrimidine-5-carboxylate;
[0048] S2. Add ethyl 4-hydroxy-2-methylthiopyrimidine-5-carboxylate (55.6 g, 0.26 mol) obtained in step S1 to 150 mL of acetonitrile, stir for 25 min, and slowly add 135 mL of PCl dropwise to the reaction solution 5 , after the dropwise addition, the reaction solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com