Hydrochloric acid dapoxetine technology impurities, preparation and use thereof
A technology of dapoxetine hydrochloride and hydrochloride, applied in the field of drug synthesis, can solve the problems such as no process impurity report yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
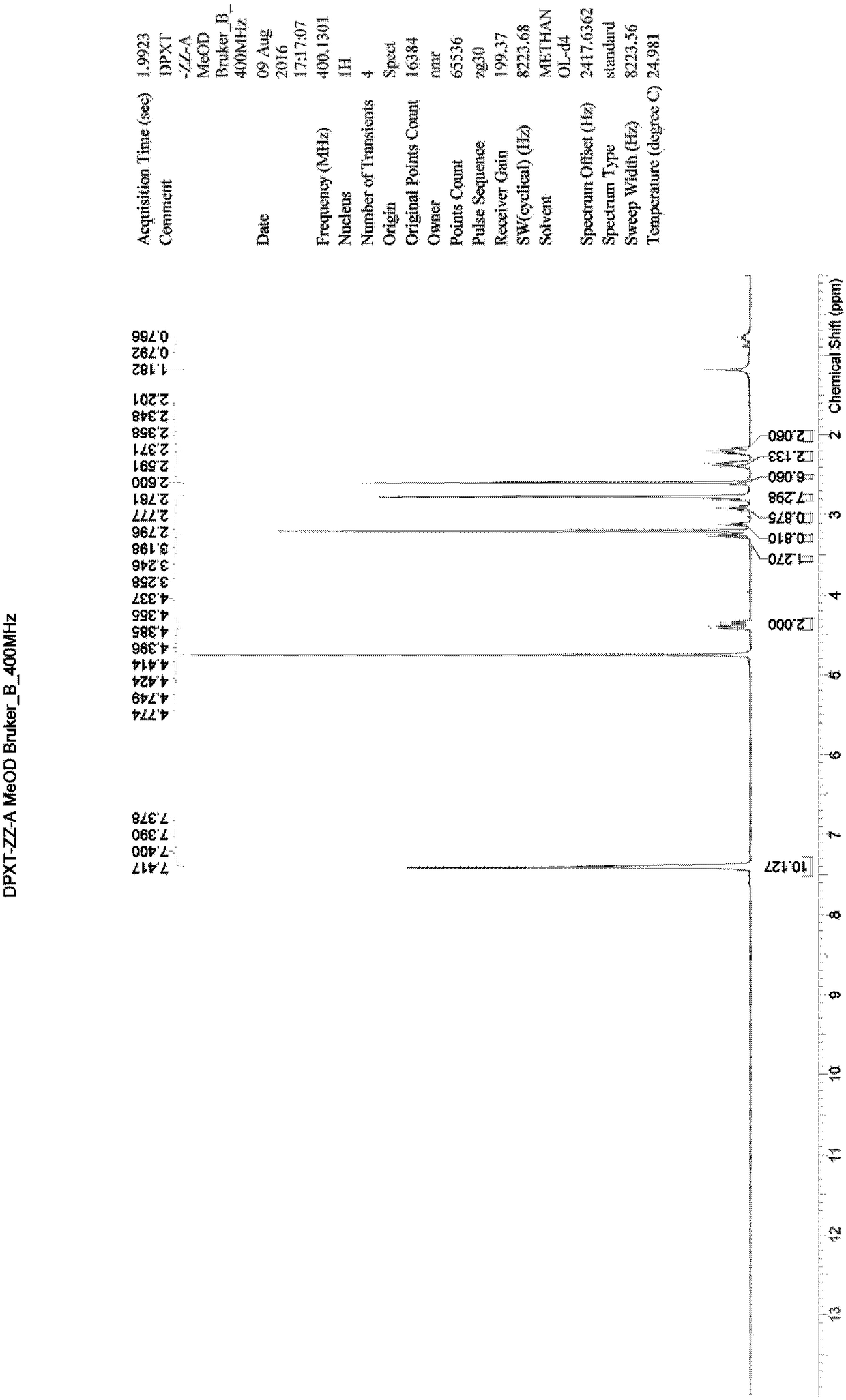
[0030] Example 1: Synthesis of 3,3'-oxybis(1-phenyl-1-propanone) (compound 2)
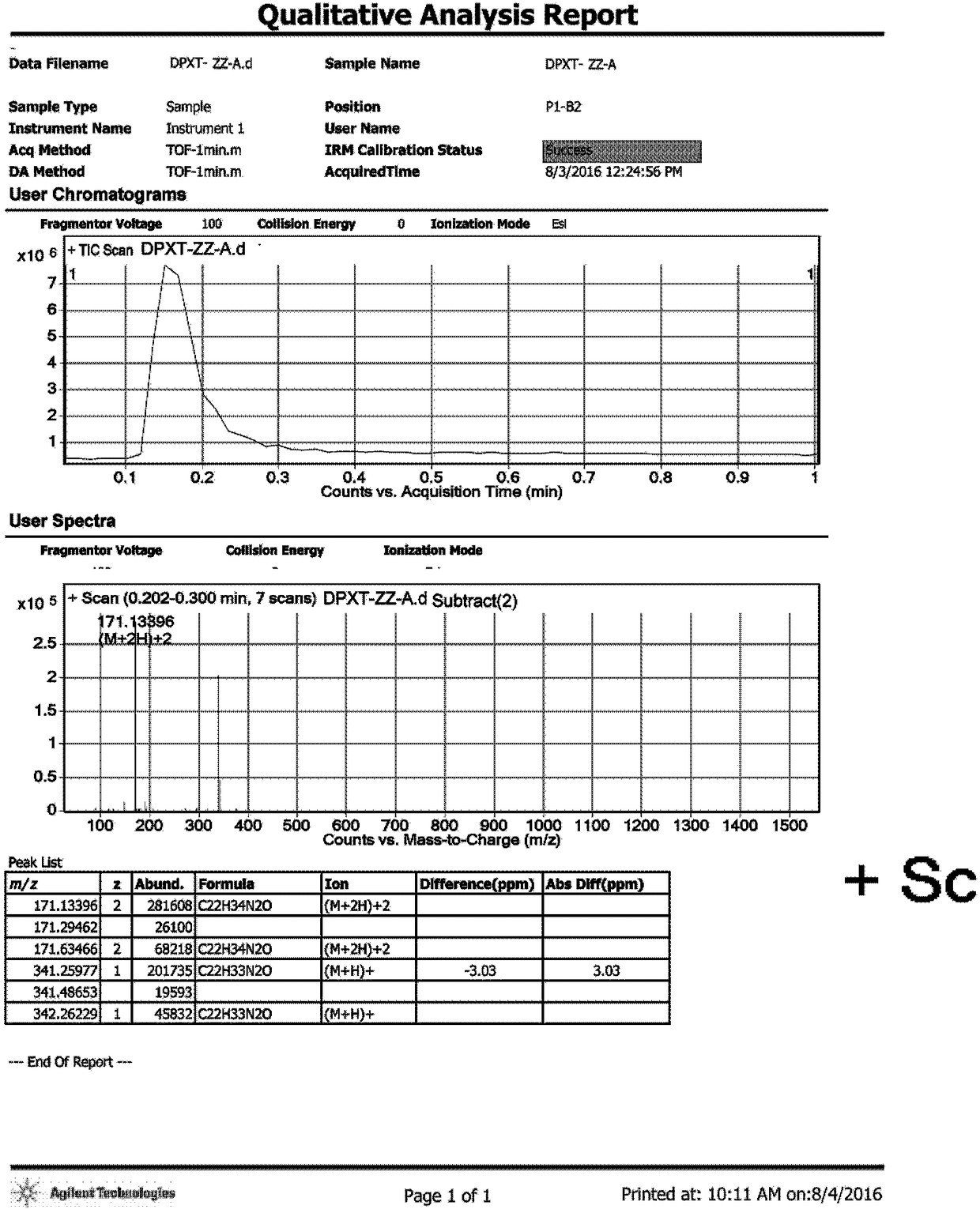
[0031] Add 100mL toluene and 20mL water into a 500mL three-necked flask, start stirring, add 20.00g SM1 (3-chloropropiophenone), 2.00g p-toluenesulfonic acid in sequence, raise the temperature to 90°C, keep stirring for 24 hours, and take samples for LCMS Detection, product generation (m / z=283[M+H] + ). Separate the liquid, collect the organic phase, control the temperature at 30-40°C, vacuum degree: -0.08 MPa, distill under reduced pressure, and distill off the solvent until no distillate is distilled to obtain 18.62 g of a yellow oily substance. The obtained crude product was separated and purified by column chromatography, the eluent ratio was: methanol: dichloromethane = 1:50, a total of 1600 mL of eluent was collected, the temperature was controlled at 30-40 ° C, vacuum degree: -0.08 MPa, and vacuum distillation , the solvent was distilled off until no distillate was distilled to obtain 2.52...
Embodiment 2
[0032] Example 2: Synthesis of 3,3'-oxybis(1-phenyl-1-propanol) (compound 3)
[0033] Add 50mL of methyl tert-butyl ether into a 250mL three-necked flask, start stirring, add 2.00g of 3,3'-oxybis(1-phenyl-1-propanone) (compound 2), cool down to 0-10°C, Slowly add 1.02 g of sodium borohydride in batches, and react overnight at room temperature after the addition is complete. A sample was taken for TLC detection (n-heptane: ethyl acetate = 8:1), and the raw material disappeared. Control the temperature at 10-20°C, slowly add 50mL of hydrochloric acid solution (1mol / L), separate the liquid, collect the organic phase, control the temperature at 30-40°C, vacuum: -0.08MPa, distill under reduced pressure, distill off the solvent until there is no distillate. 1.65 g of transparent oil was obtained, yield: 81.34%. That is, 3,3'-oxybis(1-phenyl-1-propanol) was obtained.
Embodiment 3
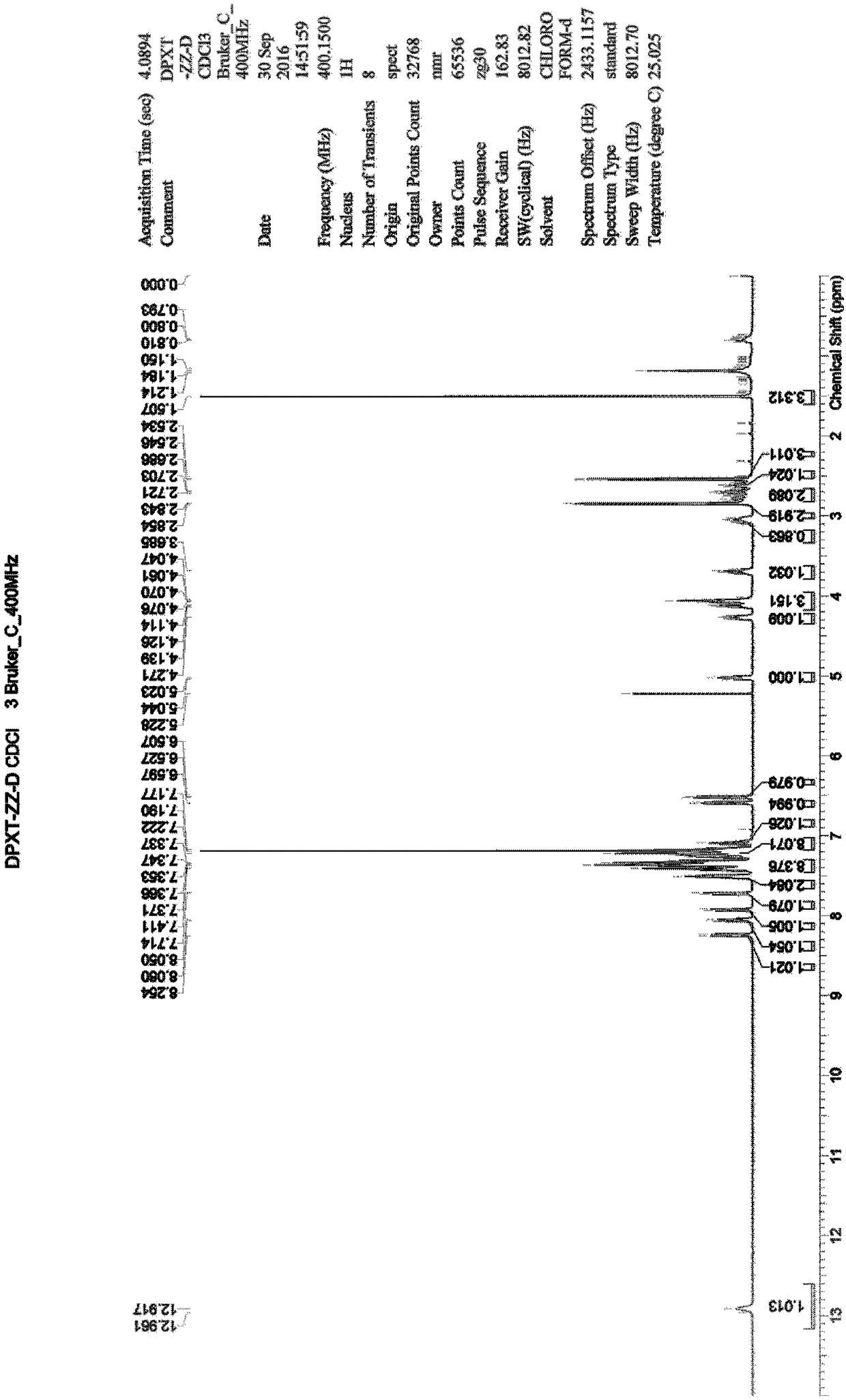
[0034] Example 3: Synthesis of 3,3'-oxybis(N,N-dimethyl-1-amphetamine) hydrochloride (impurity A)
[0035] Add 20ml of methyl tert-butyl ether to a 100mL three-necked flask, start stirring, and then add 1.16g of 3,3'-oxybis(1-phenyl-1-propanol) (compound 3), 0.98g of triethylamine , under nitrogen protection, lower the temperature to 10-20°C, start to add 1.00g of methanesulfonyl chloride dropwise, after dropping, continue to stir for 1-2 hours, take a sample for TLC detection (n-heptane: ethyl acetate = 3:2), the raw material disappear. Add 60 mL of dimethylamine tetrahydrofuran solution (2 mol / L) to the system, raise the temperature to 30°C, and stir overnight; take a sample for TLC detection (n-heptane: ethyl acetate = 4:1) and the raw material disappears. Add 60ml of purified water, separate the liquid, collect the organic phase, distill under reduced pressure, distill off the solvent until no fraction is distilled off to obtain an oily crude product, which will be purifi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com