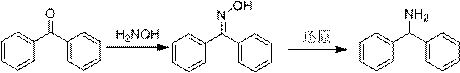
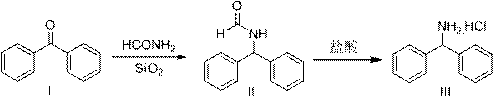
A kind of preparation method of diphenylmethylamine hydrochloride
A technology for benzophenone hydrochloride and benzophenone, applied in the field of medicine, can solve the problems of high reaction temperature, long reaction time, large amount of formamide, etc., and achieves the advantages of shortening reaction time, reducing energy consumption and improving yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of intermediate (II)
[0034] Add 100 g of benzophenone, 148 g of formamide, and 5.0 g of 200-300 mesh chromatography silica gel, raise the temperature to 185-190° C., and react for 4 hours. Sampling HPLC monitoring (area normalization method): product 96.10%, benzophenone raw material: 0.06%. Cool the reaction solution, add water, stir and disperse evenly, filter, wash and dry to obtain 112.7 g of product, yield 97.2%, HPLC purity: 97.17%.
Embodiment 2
[0035] Example 2 Preparation of intermediate (II)
[0036] Add 100 g of benzophenone, 148 g of formamide, and 10 g of 200-300 mesh chromatography silica gel, raise the temperature to 185-190° C., and react for 3 hours. Sampling HPLC monitoring (area normalization method): product 95.15%, benzophenone raw material: 0.03%. Cool the reaction solution, add water, stir and disperse evenly, filter, wash and dry to obtain 114.0 g of product, yield 98.4%, HPLC purity: 96.94%.
Embodiment 3
[0037] Example 3 Preparation of intermediate (II)
[0038] Add 100 g of benzophenone, 148 g of formamide, and 20 g of 200-300 mesh chromatography silica gel, raise the temperature to 180-185° C., and react for 3 hours. Sampling HPLC monitoring (area normalization method): product 96.43%, benzophenone raw material: 0.02%. Cool the reaction solution, add water, stir and disperse evenly, filter, wash and dry to obtain 113.4 g of product, yield 97.8%, HPLC purity: 97.34%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com