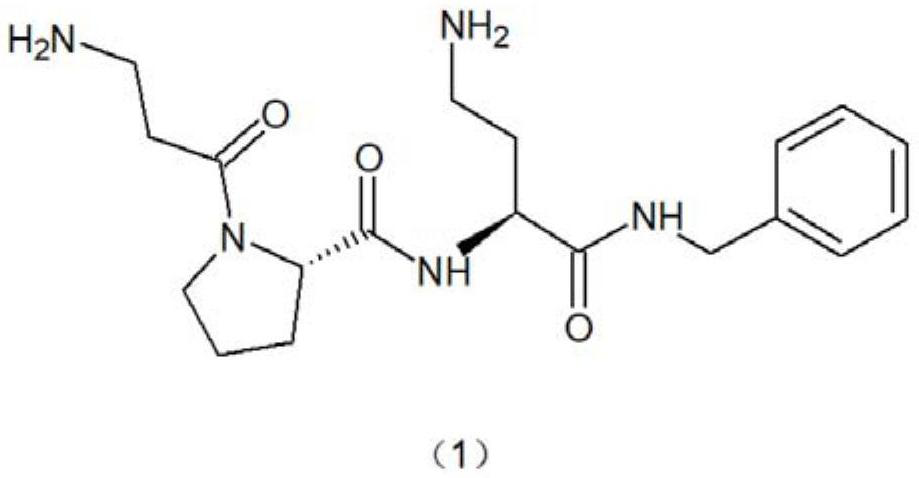
Synthesis method of snake venom-like tripeptide
A synthetic method, snake venom technology, applied in the field of polypeptide liquid phase synthesis, can solve the problems of high price of Pd/C, potential safety hazards of cosmetics, high synthesis cost, etc., and achieve the effect of market competitiveness, easy scale-up synthesis, and controllable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A synthetic method of snake venom tripeptide, comprising the following steps:
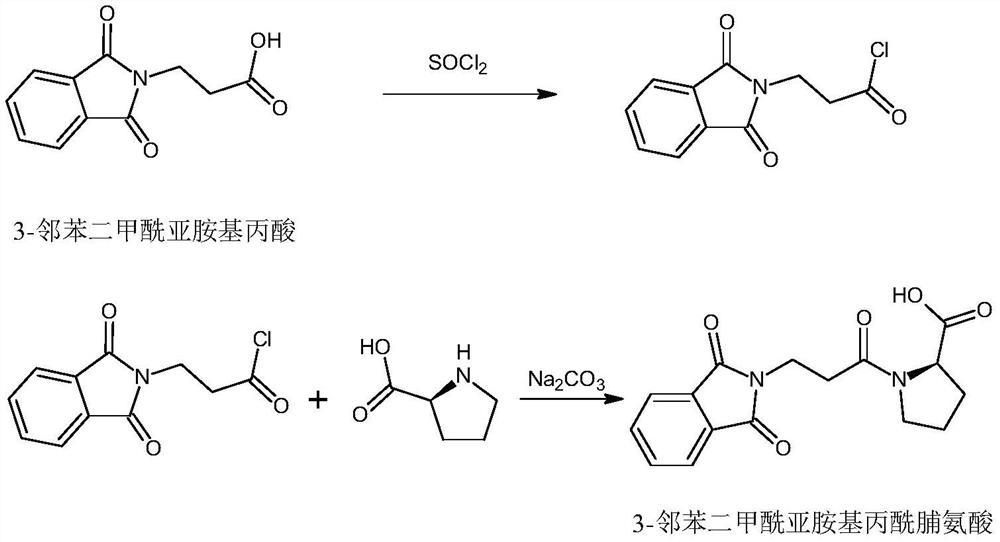
[0046] (1) Synthesis of 3-phthalimide propionyl chloride:
[0047] Put 20 grams of 3-phthalimidopropionic acid into a 500 ml reaction bottle, add 100 ml of toluene, seal it, add 40 ml of thionyl chloride, stir, stir at room temperature for 1 hour, and then heat to 60°C React for 2-3 hours, then reflux for 6-8 hours. After the reaction is finished, thionyl chloride and toluene are recovered under reduced pressure and recovered to dryness as much as possible. Inhale 100 ml of dichloromethane, stir to dissolve.
[0048] (2) Synthesis of 3-phthalimidopropionylproline:
[0049]Add 23 grams of sodium carbonate to 100 milliliters of water, stir until completely dissolved, add 15 grams of L-proline and stir, cool to below 20°C, add dropwise 3-phthalimidopropionyl chloride obtained in step (1) dichloromethane solution, reacted at room temperature, maintained the pH value at 9-10, added dropwise, ...
Embodiment 2
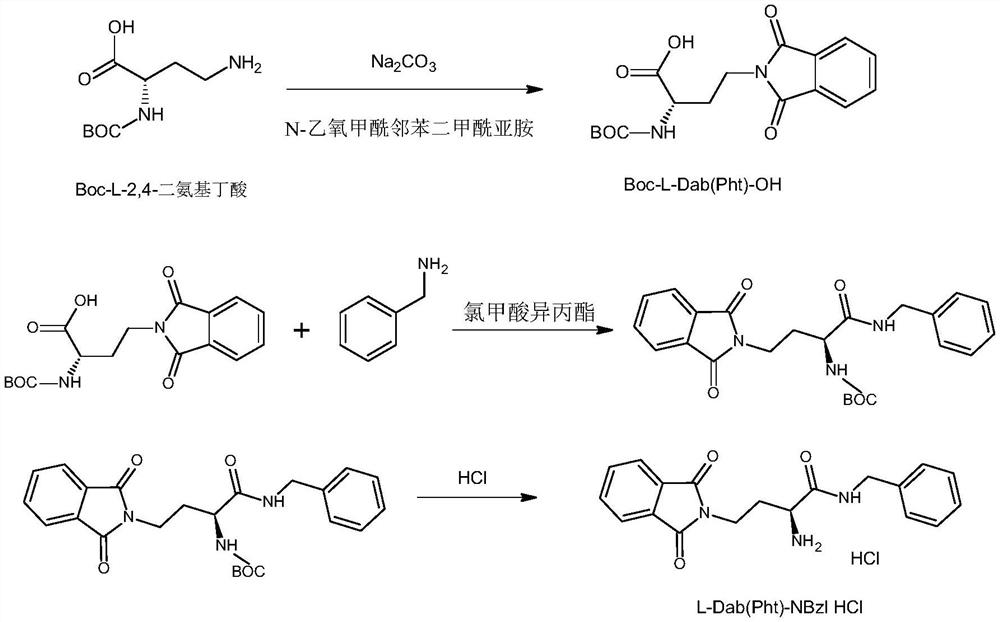
[0063] Condensation reaction:
[0064] Combine 6 grams of 3-phthalimidopropionylproline obtained in step (2) of Example 1 with the side chain phthaloyl-protected L-2,4 obtained in step (5) of Example 1 - Add 5 grams of diaminobutyrylbenzylamine hydrochloride into a 250 ml three-necked reaction flask, add 80 ml of DMF, then add 10 grams of condensing agent HBTU, and 2 ml of triethylamine for condensation reaction at room temperature, react for 4-8 hours, then add 100 milliliters of water, add 100 milliliters of ethyl acetate to extract 3 times, the ethyl acetate layer is washed with water 3 times, 0.5N hydrochloric acid 3 times, 5% sodium carbonate aqueous solution 3 times, saturated saline 3 times, then add anhydrous sodium sulfate It was dried, filtered, and concentrated to obtain 9.5 g of an oily product with a purity of 97.2% by HPLC.
[0065] Synthesis of snake venom-like tripeptide hydrochloride:
[0066] With gained free snake venom tripeptide 5 grams. Add 5 ml of hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com