Pyrazole compound preparation method
A compound and pyrazole technology, applied in the field of preparation of pyrazole compounds, can solve the problems of low production efficiency and production cost, long reaction period, serious side reactions, etc., and achieve precise control of reaction conditions, shortened reaction time, and reduced The effect of by-product formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
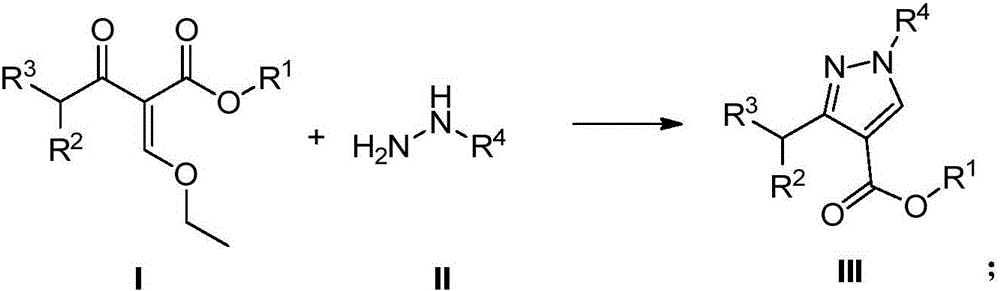
[0038] 1. Raw material preparation: Weigh compound a (31.5g, 0.142mol) to configure its xylene solution (80.0mL), put it into a container, stir it to mix evenly and seal it, and put it in raw material tank 1; weigh compound b (40 % methylhydrazine aqueous solution, 17.2g, 0.149mol, 19.8mL), put in raw material tank 2;
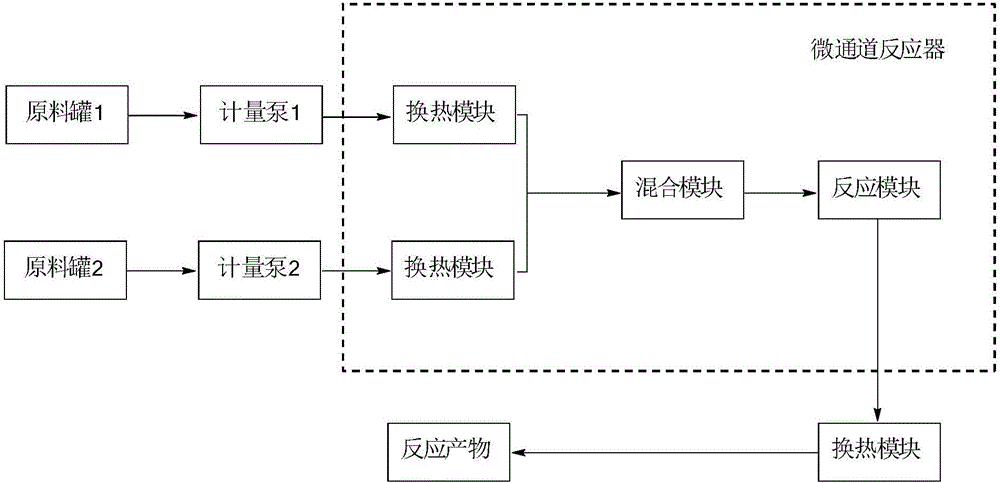
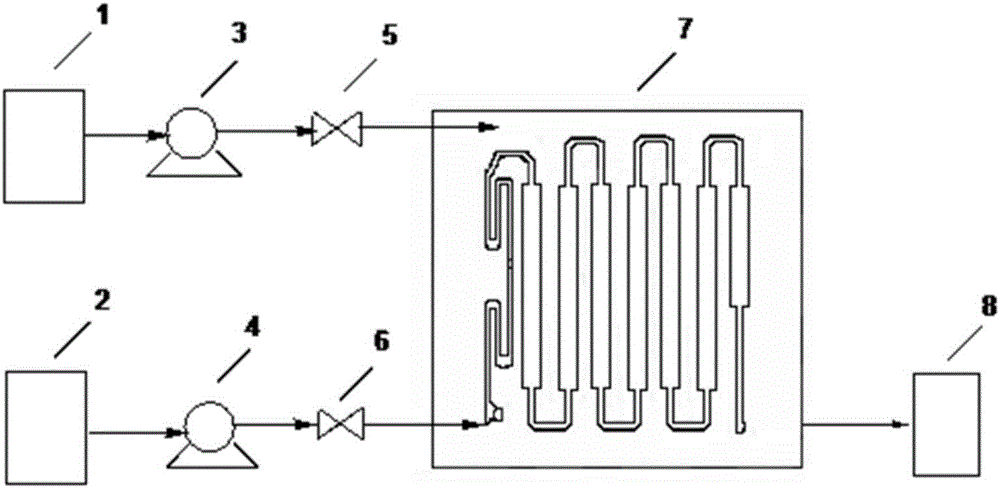
[0039] 2, utilize the device of the present invention figure 2 , according to the following steps: (1) the xylene solution of compound a in raw material tank 1 enters Corning microchannel reactor 7 by metering pump 3, and the 40% methylhydrazine aqueous solution in raw material tank 2 enters Corning microchannel reactor by metering pump 4 Channel reactor 7; (2) setting each metering pump 3 and metering pump 4 to control the xylene solution and 40% methylhydrazine aqueous solution to be 20mL / min and 5.0mL / min respectively, and the heat exchanger temperature is set to 25°C, the reaction residence time is 240s; (3) Stop valves 5 and 6 are used to pre...
Embodiment 2
[0042]
[0043] 1. Preparation of raw materials: Weigh compound d (36.2g, 0.142mol) to prepare its toluene solution (180.0mL), put it into a container, stir to make it evenly mixed and sealed, and put it in raw material tank 1; weigh compound e (phenylhydrazine , 16.9g, 0.156mol), be mixed with its toluene suspension (60.0mL), be put in the raw material tank 2;
[0044] 2, utilize the device of the present invention figure 2 , according to the following steps: (1) the toluene solution of compound d in raw material tank 1 enters Corning microchannel reactor 7 by metering pump 3, and the toluene suspension of phenylhydrazine in raw material tank 2 enters Corning microchannel reactor by metering pump 4 Channel reactor 7; (2) setting each metering pump 3 and metering pump 4 to control the toluene solution of compound d and the toluene suspension of phenylhydrazine to be 30mL / min and 10.0mL / min respectively, and the heat exchanger temperature is set to 55°C, reaction residence...
Embodiment 3
[0047]
[0048] 1. Preparation of raw materials: Weigh compound g (41.6g, 0.200mol) to prepare its toluene solution (160.0mL), put it into a container, stir to mix it evenly and seal it, and put it in raw material tank 1; weigh compound b (40% Aqueous methylhydrazine solution, 24.2g, 0.210mol, 27.8mL), was placed in the raw material tank 2;
[0049] 2, utilize the device of the present invention figure 2 , according to the following steps: (1) the toluene solution of compound g in the raw material tank 1 enters the Corning microchannel reactor 7 through the metering pump 3, and the 40% methylhydrazine aqueous solution in the raw material tank 2 enters the Corning microchannel through the metering pump 4 Reactor 7; (2) set each metering pump 3 and metering pump 4 to control the toluene solution of compound g and 40% methylhydrazine aqueous solution to be 25mL / min and 4.3mL / min respectively, and set the heat exchanger temperature to be 40°C , reaction residence time 390s; (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com