A kind of preparation method of lithium dioxalate borate
A technology of lithium bisoxalate borate and lithium bisoxalate borate, which is applied in the field of preparation of lithium bisoxalate borate, can solve the problems of difficult large-scale production, complex process, and low product purity, and increase the atomic diffusion distance , simple process, precise control of the effect of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
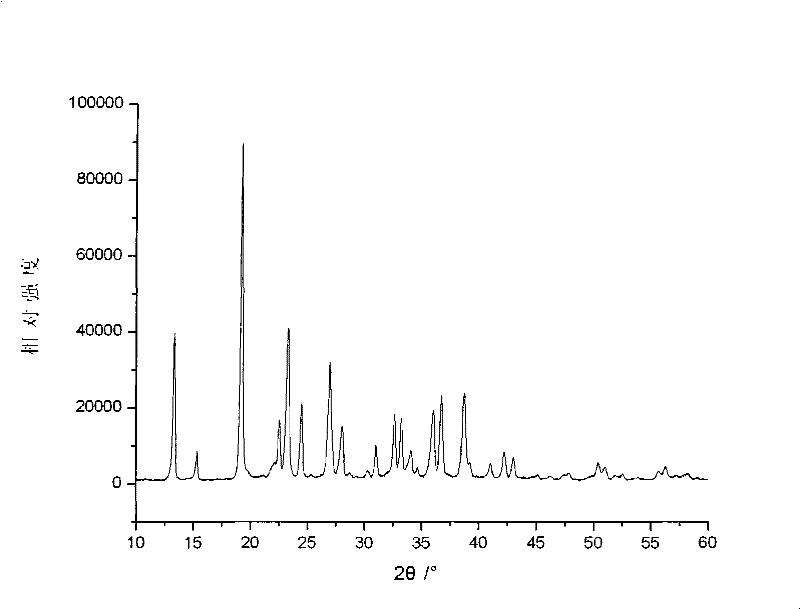
Embodiment 1
[0030] In the first step, raw materials are mixed under liquid phase conditions to form raw material slurry
[0031] 28.42 grams of analytically pure solid raw material ammonium oxalate, 13.38 grams of lithium iodide and 6.18 grams of boric acid are added in 240 grams of ethanol aqueous solution, wherein the mol ratio of solid raw material is ammonium oxalate: lithium iodide: boron=2: 1: 1, The aqueous ethanol solution is prepared according to the mass ratio of ethanol: water=1:1, the mass ratio of the solid raw material mixture to the aqueous ethanol solution is 1:5, and is stirred and mixed evenly at 40° C. to form a raw material slurry;
[0032] The second step, liquid phase reaction makes lithium bisoxalate borate slurry
[0033] Place the raw material slurry obtained in the first step in an airtight container, raise the temperature to 120°C under the condition of nitrogen atmosphere and pressure of 0.2MPa, react for 3 hours, then cool down to room temperature to obtain di...
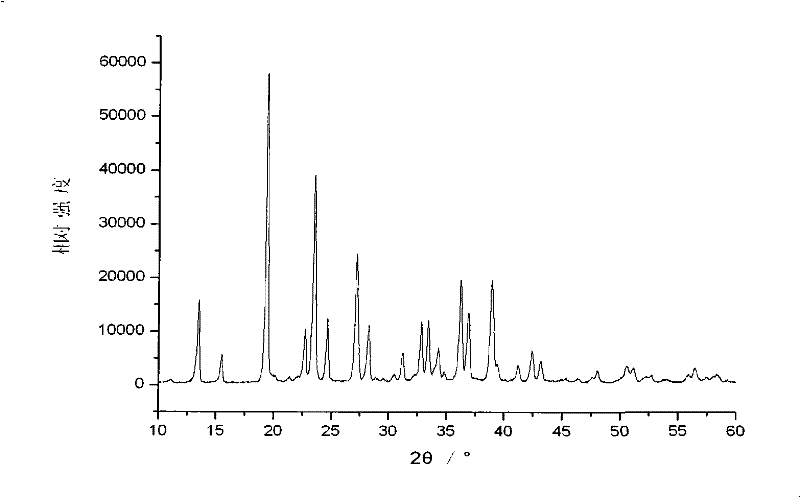
Embodiment 2
[0038] In the first step, raw materials are mixed under liquid phase conditions to form raw material slurry
[0039] 29.84 grams of analytically pure solid raw material ammonium oxalate, 13.38 grams of lithium iodide and 3.48 grams of diboron trioxide are added in 46.7 grams of ethanol aqueous solution, wherein the mol ratio of solid raw material is ammonium oxalate: lithium iodide: boron=2.1:1 : 1, the aqueous ethanol solution is prepared according to the mass ratio of ethanol: water=3: 1, the mass ratio of the solid raw material mixture to the aqueous ethanol solution is 5: 5, and is stirred and mixed evenly at 60° C. to form a raw material slurry;
[0040] The second step, liquid phase reaction makes lithium bisoxalate borate slurry
[0041] Put the raw material slurry obtained in the first step in an airtight container, raise the temperature to 180°C under the condition of argon atmosphere and pressure of 1MPa, react for 1 hour, then cool down to room temperature to obtain b...
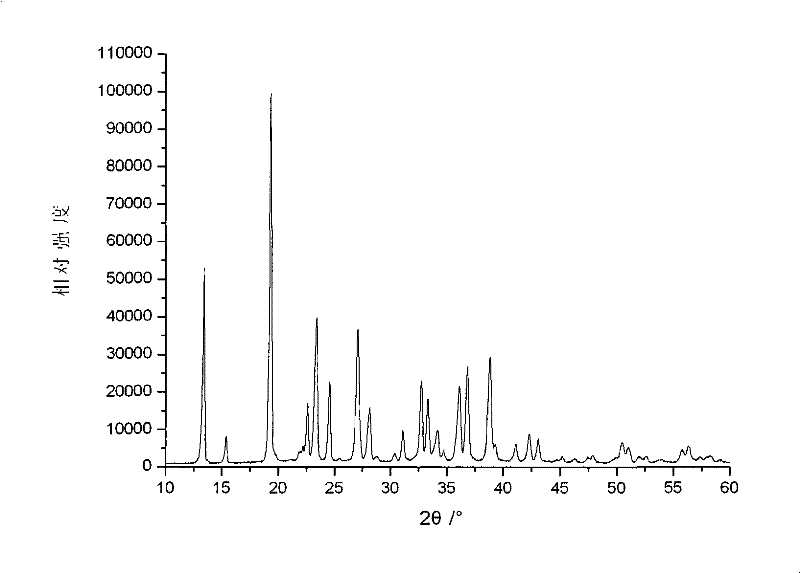
Embodiment 3
[0046] In the first step, raw materials are mixed under liquid phase conditions to form raw material slurry
[0047] 29.13 grams of analytically pure solid raw material ammonium oxalate, 13.38 grams of lithium iodide and 3.48 grams of diboron trioxide are added in 138 grams of ethanol aqueous solution, wherein the mol ratio of solid raw material is ammonium oxalate: lithium iodide: boron=2.05: 1 : 1, the aqueous ethanol solution is prepared according to the mass ratio of ethanol: water=2: 1, the mass ratio of the solid raw material mixture and the aqueous ethanol solution is 2: 5, stirred and mixed evenly at 50° C. to form a raw material slurry;
[0048] The second step, liquid phase reaction makes lithium bisoxalate borate slurry
[0049] Put the raw material slurry obtained in the first step in an airtight container, raise the temperature to 150°C under the condition of nitrogen atmosphere and pressure of 0.4MPa, react for 2 hours, then cool down to room temperature, and obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com