Method for preparing retegravir intermediate by using continuous flow reactor
A technology of remdesivir and intermediates, which is applied in the field of remdesivir synthesis, can solve problems such as difficult reaction conditions, unfavorable pilot production, and low reaction yield, and achieve precise control of reaction conditions, shortened reaction time, and reduced The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
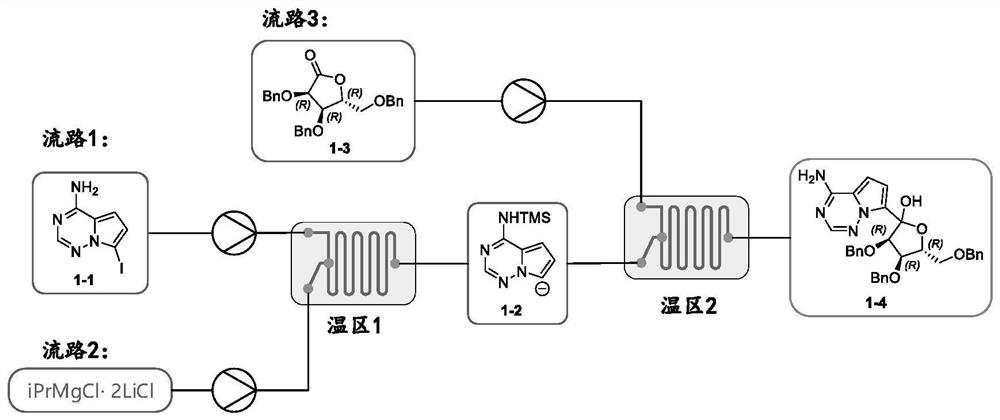
[0038] 1. Flow path 1: Add 120g of 7-iodopyrrole[2,1-f][1,2,4]triaza-4-amine 1-1 into 2600mL tetrahydrofuran, cool down to -5~- At 10°C, add 260g of phenylmagnesium chloride dropwise, finish adding in 30 minutes, keep warm for 30 minutes, add 55g of trimethylchlorosilane dropwise, stir for 60 minutes, then add 260g of phenylmagnesium chloride to form a solution of about 0.14mol / L , as a backup channel 1.
[0039] 2. Flow path 2: Dilute 500 mL of 1.2 mol / L isopropylmagnesium chloride lithium chloride solution with 400 mL of anhydrous THF to prepare a solution of about 0.72 mol / L, which is used as flow path 2 for later use.
[0040] 3. Flow path 3: Add 240g of (3R,4R,5R)-3,4-dibenzyl-5-(benzylmethyl)dihydrofuran-2(3H)-one 1-3 to 1100mL In the tetrahydrofuran solution of neodymium trichloride (0.58mol / L), stir and dissolve and add 200mL of tetrahydrofuran to form a tetrahydrofuran solution, which is used as the flow path 3 for later use.
[0041] 4. Connect the reac...
Embodiment 2
[0050]
[0051] 1. Flow path 1: Add 75.6g of 7-bromopyrrole[2,1-f][1,2,4]triaza-4-amine 2-1 into 2000mL tetrahydrofuran, cool down to -15~ -20°C, add 200g of phenylmagnesium chloride dropwise, finish adding in 30 minutes, keep warm for 30 minutes, add 42.3g of trimethylchlorosilane dropwise, stir for 60 minutes, then add 200g of phenylmagnesium chloride, and configure it to about 0.14mol / L solution, as solution A.
[0052] Dilute 400 mL of a 1.2 mol / L ethylmagnesium bromide lithium chloride solution with 267 mL of anhydrous THF to prepare a solution of about 0.72 mol / L as solution B.
[0053] Keep at -15~-20°C, slowly drop solution B into solution A, stir for 60 minutes, and use it as flow path 1 for later use.
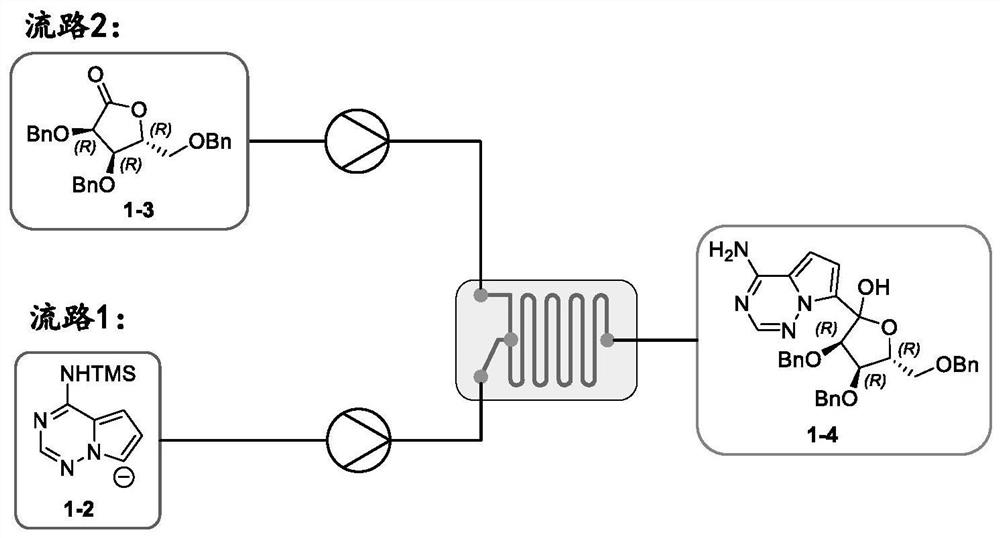
[0054] 2. Flow path 2: Add 184.6g of (3R,4R,5R)-3,4-dibenzyl-5-(benzylmethyl)dihydrofuran-2(3H)-one 1-3 to 846mL lanthanum trichloride tetrahydrofuran solution (0.58mol / L), stirring and dissolving and adding 154mL tetrahydrofuran to configure a tetrahydrofuran so...
Embodiment 3
[0064]
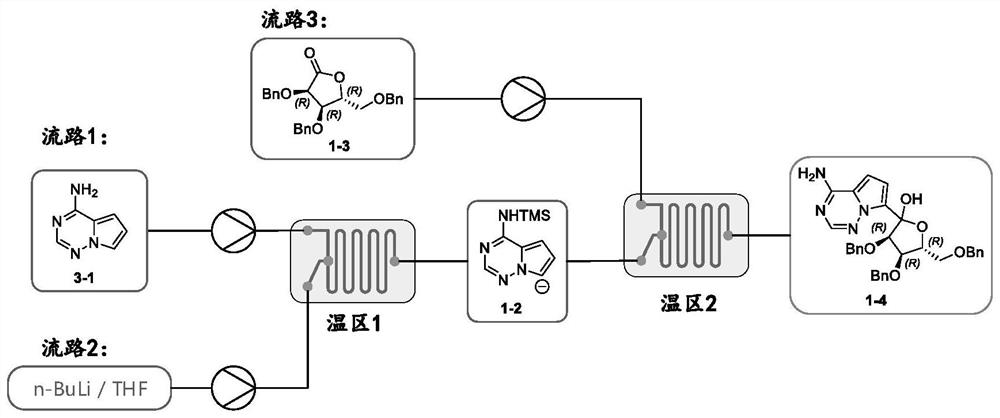
[0065] 1. Flow path 1: Add 100g of pyrrole[2,1-f][1,2,4]triaza-4-amine 1-1 into 1500mL tetrahydrofuran, cool down to -5~0℃, add 86.5 g tetramethylethylenediamine, slowly dropwise add 81 g of trimethylchlorosilane, stir for 60 minutes, and use it as flow path 1 for later use.
[0066] 2. Flow path 2: 1000 mL of 2.5 mol / L n-butyllithium tetrahydrofuran solution is used as flow path 2 for standby.
[0067] 3. Flow path 3: Add 312g of (3R,4R,5R)-3,4-dibenzyl-5-(benzylmethyl)dihydrofuran-2(3H)-one 1-3 to 1435mL In a tetrahydrofuran solution of neodymium trichloride (0.52 mol / L), stir at 20-25° C. for 60 minutes, and use it as flow path 3 for later use.
[0068] 4. Connect the reactor pipeline, such as image 3 shown. Connect flow path 1 and flow path 2 to reaction module 1, set temperature zone 1, as a negative ion module; connect the outlet of module 1 and flow path 3 to reaction module 1, set temperature zone 2, as a coupling reaction module.
[0069] 5. Set the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com