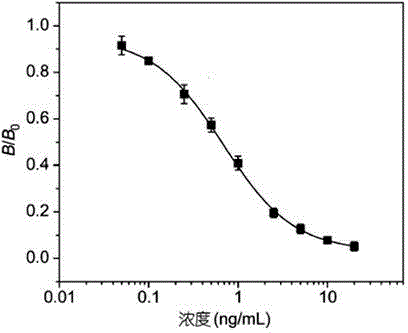
Time-resolved fluoroimmunoassy kit for detecting race-quantity mebendazole residual
A time-resolved fluorescence and mebendazole technology, which is applied in the field of immunological detection, can solve the problems of low sensitivity and narrow emission spectrum band, and achieve the effects of high sensitivity, reduced operation steps and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation of embodiment 1 kit components
[0058] 1. Antigen synthesis
[0059] a. Acylate the alcoholic hydroxyl group in the molecular structure of mebendazole with glutaraldehyde anhydride to obtain the hapten of mebendazole
[0060] b. Dissolve 1 g of the hapten of the mebendazole drug in 10 ml of 0.5 M sodium hydroxide solution.
[0061] c. Dissolve 1 g of carbodiimide in 5 ml of pure water, then add it to the hapten solution and stir at room temperature for 3 hours.
[0062] d. Dissolve 10 g of the carrier protein ovalbumin (OVA) or bovine serum albumin (BSA) in 35 mL of pH9.6 carbonate buffer solution, and add the carrier protein dropwise to the hapten and react overnight at 4°C.
[0063] e. The artificial antigen obtained after the reaction was dialyzed against 0.1M PBS buffer for 5 days, and the buffer was changed 4 times a day; the obtained purified artificial antigen was concentrated by ultrafiltration or lyophilized for storage.
[0064] 2. Preparat...
Embodiment 2
[0077] Example 2 Formation of Time-Resolved Fluorescence Immunoassay Kit for Detection of Trace Mebendazole Residues
[0078] A time-resolved immunoassay kit for detecting mebendazole was constructed to include the following components:
[0079] (1) ELISA plate coated with Mebendazole drug antigen
[0080] (2) Eu 3+ Labeled mebendizumab working solution
[0081] (3) Mebendazole standard with a concentration of 1 μg / mL
[0082] (4) Washing buffer: 0.05mol / LTris-HCl, pH8.0, 0.9%NaCl, 0.04%Tween20.
[0083] (5) Standard diluent: 0.05mol / LTris-HCl, pH 8.0, 0.9%NaCl buffer.
[0084] (6) Fluorescence enhancement solution: 0.1mol / L potassium hydrogen phthalate-acetic acid buffer solution, containing 15umol / L β-NTA, 0.1% TritonX-100.
Embodiment 3
[0085] The detection of mebendazole drug residue in the sample of embodiment 3
[0086] 1. Sample pretreatment
[0087] a. Tissue samples: Homogenize lean meat or visceral tissue samples with a homogenizer, accurately weigh 4g of the sample, add 4mL of ethyl acetate, shake for 10 minutes, centrifuge at room temperature for 15 minutes at 4000rpm / min, take out 2mL of the upper layer, and place it under nitrogen flow Dry at 50-60°C, add 1mL of n-hexane to dissolve the dried residue, add 1mL of standard diluent, shake vigorously for 1min, centrifuge at room temperature at 4000rpm / min for 5min, remove the upper oil phase, and take 50μl of the lower aqueous phase for measurement.
[0088] b. Urine: Take 2mL of urine into a centrifuge tube, add 4mL of ethyl acetate, shake for 5 minutes, centrifuge at 4000rpm / min at room temperature for 5 minutes, take out 2mL of the upper layer, dry it at 50-60°C under nitrogen flow, and add 1mL of standard The diluent was shaken and dissolved for 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| full width at half maximum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com