Preparation method of mebendazole chewable tablets
A technology of mebendazole and chewable tablets, which is applied in the field of medicine, can solve problems such as difficulties in drug treatment, achieve the effects of effectively stabilizing pain, avoiding side effects, and improving use value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
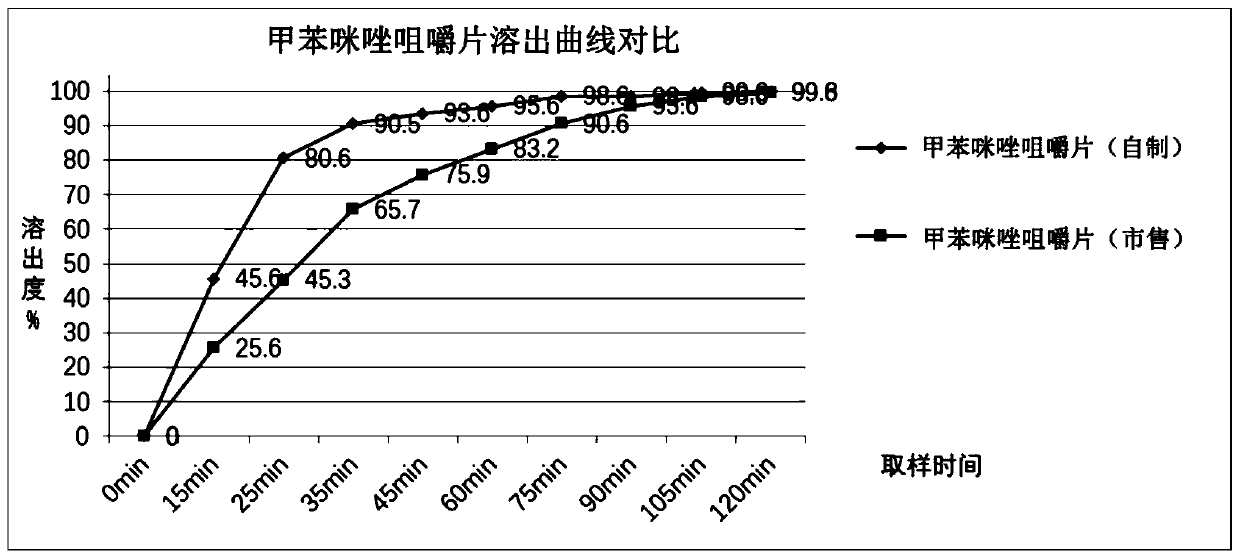
Image
Examples
Embodiment 1
[0049] 1. Prescription
[0050]
[0051] 2. Process
[0052]Weigh the prescribed amount of mebendazole, mannitol, lactose, crospovidone, and aspartame, add them into a wet granulator, add an appropriate amount of purified water, tartrazine, and sunset yellow, and make a suitable soft material. Granules, pump the prepared wet granules into the fluidized bed, and dry until the moisture content is ≤4%.
[0053] After the above granules are crushed and granulated by a granulator, the prescription amount of essence and magnesium stearate is added, mixed evenly, and compressed into tablets.
Embodiment 2
[0055] 1. Prescription
[0056]
[0057] 2. Process
[0058] Weigh the prescribed amount of mebendazole, mannitol, lactose, crospovidone, and aspartame, add to a wet granulator, add an appropriate amount of povidone K30 aqueous solution, sunset yellow, and tartrazine to make a suitable soft drink. Material, granulation, pump the prepared wet granules into the fluidized bed, and dry until the moisture content is ≤4%.
[0059] After the above granules are crushed and granulated by a granulator, the prescription amount of essence and magnesium stearate is added, mixed evenly, and compressed into tablets.
Embodiment 3
[0061] 1. Prescription
[0062]
[0063]
[0064] 2. Process
[0065] Weigh the prescribed amount of mebendazole, mannitol, lactose, sucrose, crospovidone, aspartame, add to the wet granulator, add an appropriate amount of povidone K30 aqueous solution, sunset yellow, tartrazine, and make a suitable The soft material is granulated, and the prepared wet granules are pumped into the fluidized bed, and dried until the moisture content is ≤4%.
[0066] After the above granules are crushed and granulated by a granulator, the prescription amount of essence and magnesium stearate is added, mixed evenly, and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com