Mebendazole lipid compound, preparation method and application
A technology of mebendazole lipid and mebendazole, which is applied in the field of mebendazole lipid complex and its preparation, can solve the problems of demulsification, emulsion droplet aggregation, low drug loading concentration, etc., and achieve the effect of improving fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
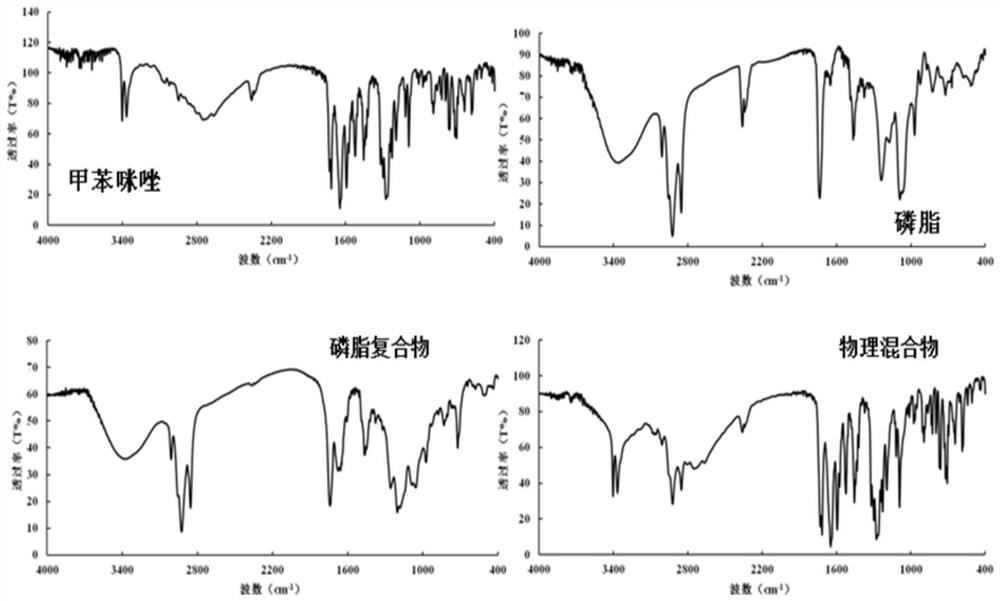
[0038] The preparation method of the above-mentioned Mbz lipoplex, the steps are as follows:
[0039] a. Weigh the mebendazole and the lipid material respectively according to the composite ratio, add the organic solvent to dissolve respectively, and mix the above two evenly;
[0040] b. After compounding at a suitable temperature for a period of time, the organic solvent is removed and vacuum-dried to obtain the mebendazole-lipid complex.
[0041] Preferably, the organic solvent is selected from one of methylene chloride, methanol, ethanol, benzyl alcohol, acetone, ethyl acetate, tetrahydrofuran, petroleum ether, tert-butanol, n-octanol, dimethylformamide or Various; preferably, one or more selected from dichloromethane, methanol, tetrahydrofuran, and acetone.
[0042] Preferably, one or more of formic acid, glacial acetic acid, sulfuric acid, trifluoroacetic acid, phosphoric acid and trinitrobenzenesulfonic acid are added to the organic solvent.
[0043] Preferably, the me...
Embodiment 1
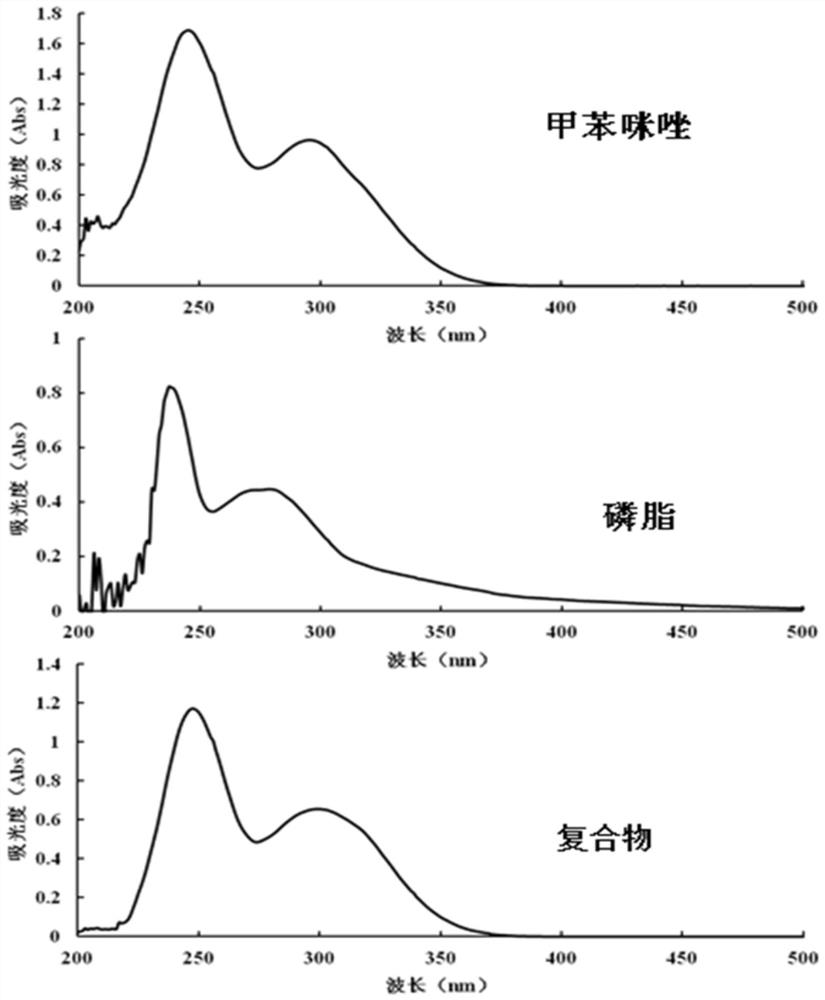
[0054] Example 1 Preparation of mebendazole phospholipid complex containing soybean lecithin in different proportions
[0055] Weigh 0.1 g of 10 parts of mebendazole independently into conical flasks, add 100 ml of methanol solution (containing 10% formic acid, v / v), and fully dissolve. Separately weigh soybean lecithin 0.27, 0.54, 0.81, 1.08, 1.35, 1.62, 1.89, 2.16, 2.43, 2.70g into a beaker, add 20ml of dichloromethane to dissolve completely, then add it into the mebendazole solution in order, and ultrasonicate at 20°C 2 hours. Transfer to a rotary evaporator, remove the solvent by rotary evaporation at 40°C, and dry under reduced pressure at room temperature for 12 hours to obtain 10 groups of composite powders with a molar ratio of mebendazole:phospholipids of 1:1-1:10.
Embodiment 2
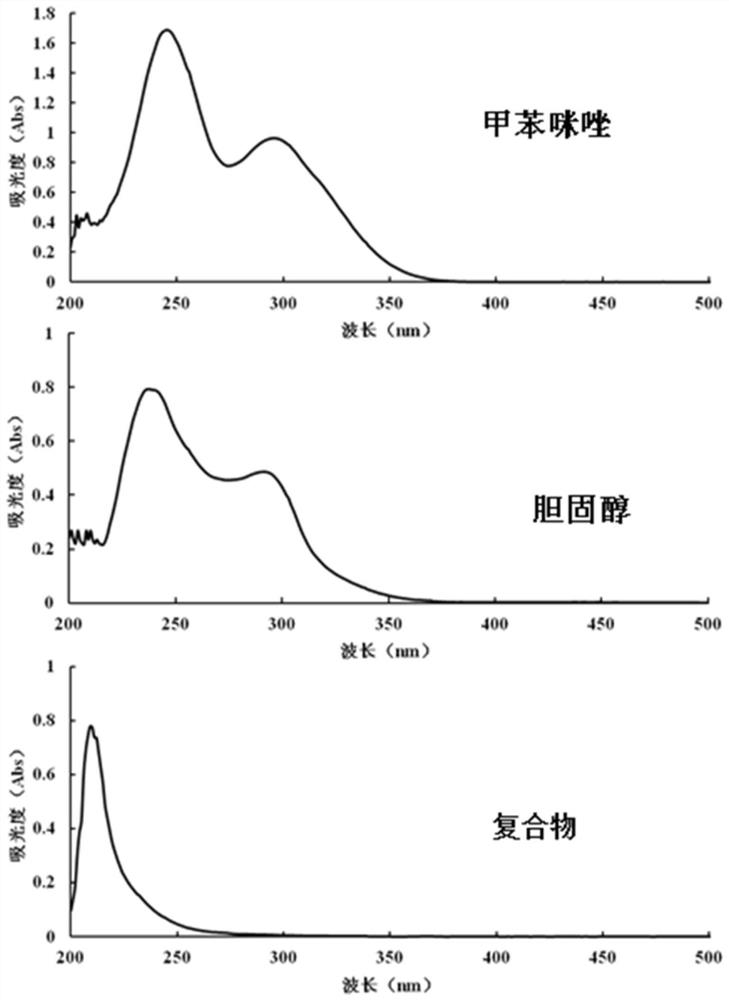
[0056] Example 2 Preparation of Mebendazole Lipid Complex Containing Different Proportions of Cholesterol
[0057] Weigh 0.1 g of 10 parts of mebendazole independently into conical flasks, add 100 ml of methanol (containing 1% trifluoroacetic acid, v / v), and fully dissolve. Separately weigh 0.13, 0.26, 0.39, 0.52, 0.65, 0.78, 0.91, 1.04, 1.17, and 1.30g of cholesterol into a beaker, add 20ml of tetrahydrofuran to dissolve completely, then add it to the mebendazole solution in sequence, and ultrasonicate for 1 hour at 20°C Let stand for 12 hours. Transfer to a rotary evaporator, remove the solvent by rotary evaporation at 500°C, and dry under reduced pressure at room temperature for 12 hours to obtain 10 groups of composite powders with a molar ratio of mebendazole:cholesterol of 1:1-1:10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com