Implant and preparation method thereof
A technology of implants and release regulators, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as difficult application, high price, and complicated structure of twin-screw extruders question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
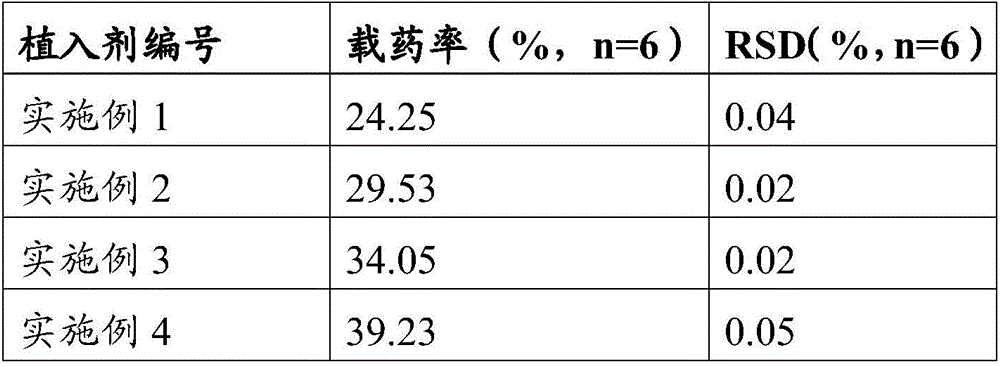
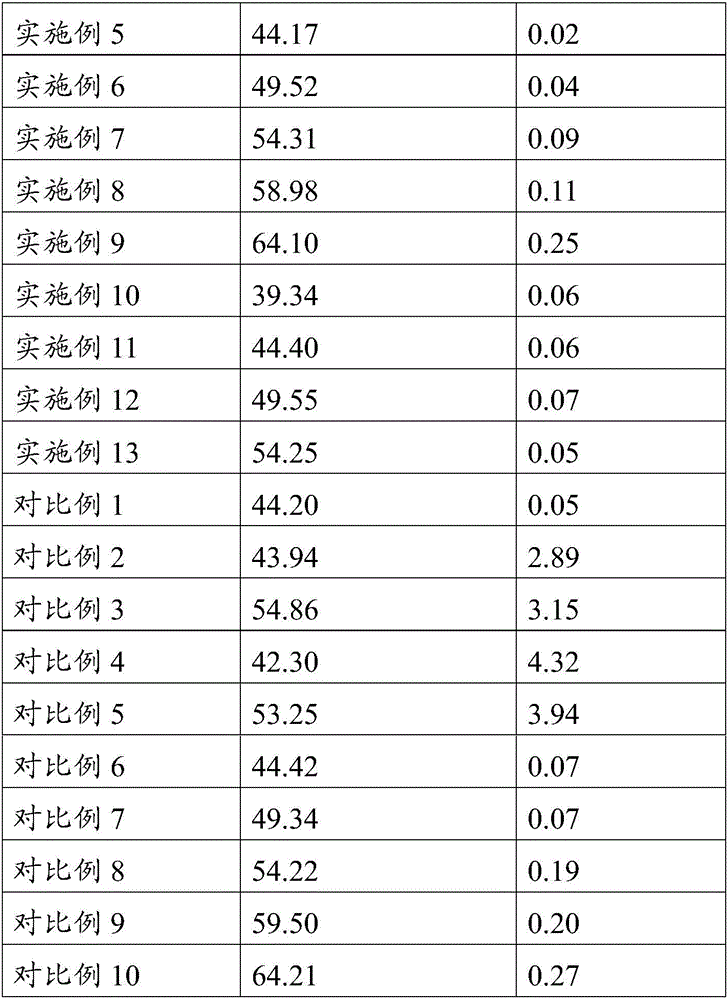
[0060] An embodiment of the implant of the present invention, the preparation raw material of the implant described in this embodiment contains the following components by weight: 26 parts of poorly water-soluble / slightly soluble drugs, 64 parts of poorly water-soluble polymers, release 10 parts of regulator; wherein the water-insoluble / slightly soluble drug is aripiprazole, and the water-insoluble polymer is PLA (the weight average molecular weight of the PLA is 25kDa, the viscosity is 0.22dL / g, and the The PLA has a terminal ester group), and the release regulator is lauric acid.
[0061] The preparation method of implant described in the present embodiment is:
[0062] (1) Pulverize the water-insoluble polymer with a feather-type pulverizer, and collect particles no larger than 40 meshes by sieving;
[0063] (2) dissolving the release modifier and the poorly water-soluble / slightly soluble drug in a mixture of tetrahydrofuran and absolute ethanol to obtain a solution;
[0...
Embodiment 2
[0068] An embodiment of the implant of the present invention, the preparation raw material of the implant described in this embodiment contains the following components by weight: 30 parts of poorly water-soluble / slightly soluble drugs, 62 parts of poorly water-soluble polymers, release 8 parts of regulators; wherein the water-insoluble / slightly soluble drug is entecavir, and the water-insoluble polymer is PLA (the PLA is a mixture of PLA with carboxyl-terminated PLA and PLA with ester-terminated PLA, and has carboxyl-terminated PLA) The mass ratio of PLA and PLA with terminal ester group is 295:300, and the weight average molecular weight of described PLA with carboxyl group and PLA with terminal ester group is 30kDa, and the viscosity is 0.28dL / g), and the release modifier is PEG600.
[0069] The preparation method of implant described in the present embodiment is:
[0070] (1) Pulverize the insoluble polymer with a blade mill, and collect particles no larger than 60 meshes...
Embodiment 3
[0076] An embodiment of the implant of the present invention, the preparation raw material of the implant described in this embodiment contains the following components by weight: 35 parts of poorly water-soluble / slightly soluble drugs, 59 parts of poorly water-soluble polymers, release 6 parts of regulators; wherein the insoluble / slightly soluble drug in water is Anastrozole, and the insoluble polymer in water is PLGA (the mol ratio of lactide and glycolide is 90:10, and the weight-average molecular weight is 35kDa , a viscosity of 0.36dL / g, with a terminal ester group), the release modifier is composed of palmitic acid and PEG800, and the mass ratio of palmitic acid to PEG800 is 42:18.
[0077] The preparation method of implant described in the present embodiment is:
[0078] (1) Pulverize the water-insoluble polymer with a feather-type pulverizer, and collect particles no larger than 80 meshes by sieving;
[0079] (2) dissolving the release regulator and the poorly water-s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com