Tamsulosin sustained-release pellet preparation and preparation method thereof
A technology of sustained-release pellets and tamsulosin, which is applied in pharmaceutical formulations, medical preparations of non-active ingredients, urinary system diseases, etc., can solve the problems of difficult control of drug release rate and high requirements for coating parameters, and achieve release Good uniformity, simple preparation process and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Tamsulosin Hydrochloride Sustained Release Capsules (5000 capsules)
[0104] 1) Sucrose core 605g
[0105] 2) Drug-loaded slow-release layer
[0106] Tamsulosin Hydrochloride 1g
[0107] Cellulose acetate 33.75g
[0108] Tween 80 7.5g
[0109] 3) Coating layer
[0110] Udrake L100-55 11.25g
[0111] Sodium Lauryl Sulfate 7.5g
[0112] Weigh the prescription amount of 2) each component in the drug-loaded sustained-release layer, dissolve it in about 600g of 95% ethanol solution to prepare the drug solution,
[0113] Weigh 3) each component of the coating layer and about 500g of purified water, and prepare the coating solution with the above auxiliary materials.
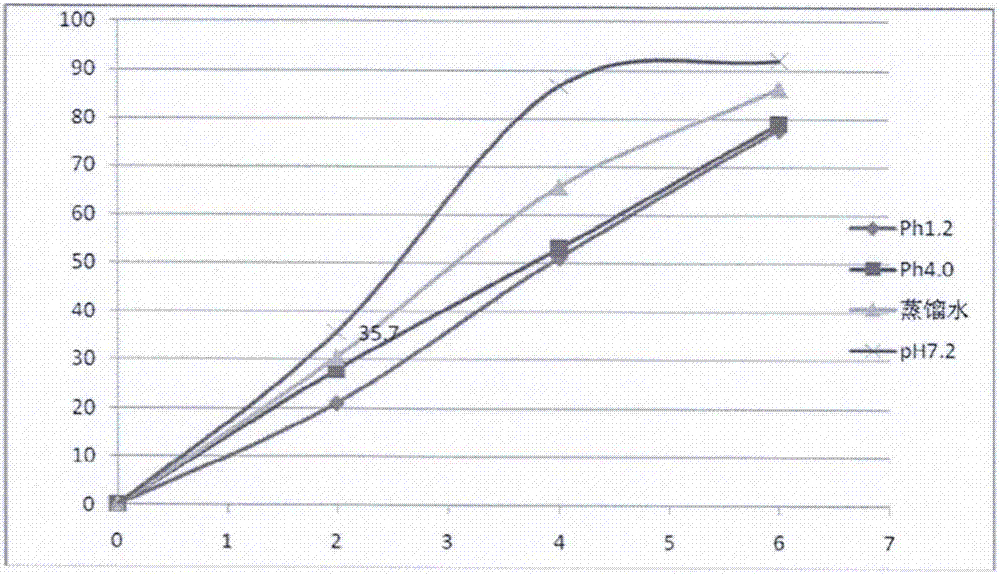
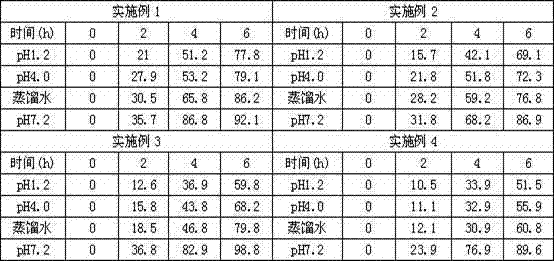
[0114]Put the blank pellet core in the fluidized bed, control the temperature of the material at 30-35°C, adjust the fluidized state, apply the medicine for about 2 hours, dry for 10 minutes after the medicine is applied, take out the upper pill and sieve, the mesh size of the sieve is 18-30 mesh, take t...
Embodiment 2
[0117] Tamsulosin Hydrochloride Sustained Release Capsules (5000 capsules)
[0118] 1) Sucrose core 505g
[0119] 2) Drug-loaded slow-release layer
[0120] Tamsulosin Hydrochloride 1g
[0121] Ethylcellulose 101g
[0122] Sodium Lauryl Sulfate 7.5g
[0123] ATBC (Acetyl Tributyl Citrate) 3.5g
[0124] 3) Coating layer
[0125] Udrake L100-55 9g
[0126] Sodium Lauryl Sulfate 7.5g
[0127] TEC 4g
[0129] Preparation
[0130] Step 1: Weigh each component according to the formula, dissolve each component of the drug-loaded sustained-release layer in about 500g of 95% ethanol to prepare a drug solution, apply the drug in a fluidized bed, and control the temperature of the material at 30-55°C to prepare on the pill,
[0131] Step 2: Sieve the upper pills, the mesh number of the sieve is 15-30 mesh,
[0132] Step 3: Take about 550g of ethanol to dissolve the components of the coating layer; place the sieved upper pills in a fluidized bed, co...
Embodiment 3
[0136] Tamsulosin Hydrochloride Sustained Release Capsules (5000 capsules)
[0137] 1) Sucrose core 660g
[0138] 2) Drug-loaded slow-release layer
[0139] Tamsulosin Hydrochloride 10g
[0140] Sodium Alginate 300g
[0141] 3) Coating layer
[0142] Udrake L100-55 10g
[0143] TEC 5g
[0144] 4) Micropowder silica gel 4g
[0146] Preparation
[0147] Step 1: Weigh each component according to the formula, dissolve each component of the drug-loaded slow-release layer in about 300g ethanol to prepare a drug solution, apply the drug in a fluidized bed, and control the temperature of the material at 40-45°C to prepare the drug solution. pill,
[0148] Step 2: Add micropowder silica gel to 2 times the water, spray it in after homogenizing with a high-speed homogenizer for 5 minutes, dry it for 5 minutes, take it out,
[0149] Step 3: Sieve the upper pill, the mesh number of the sieve is 15-30 mesh,
[0150] Step 4: Take about 100g of ethanol to d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com