Modified release tamsulosin tablets
A tamsulosin and tablet technology, which is applied in the field of monolithic drug tablets, capsules, and unit dosage forms, to treat symptoms of benign prostatic hyperplasia, and can solve problems such as food effects that have not been proposed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
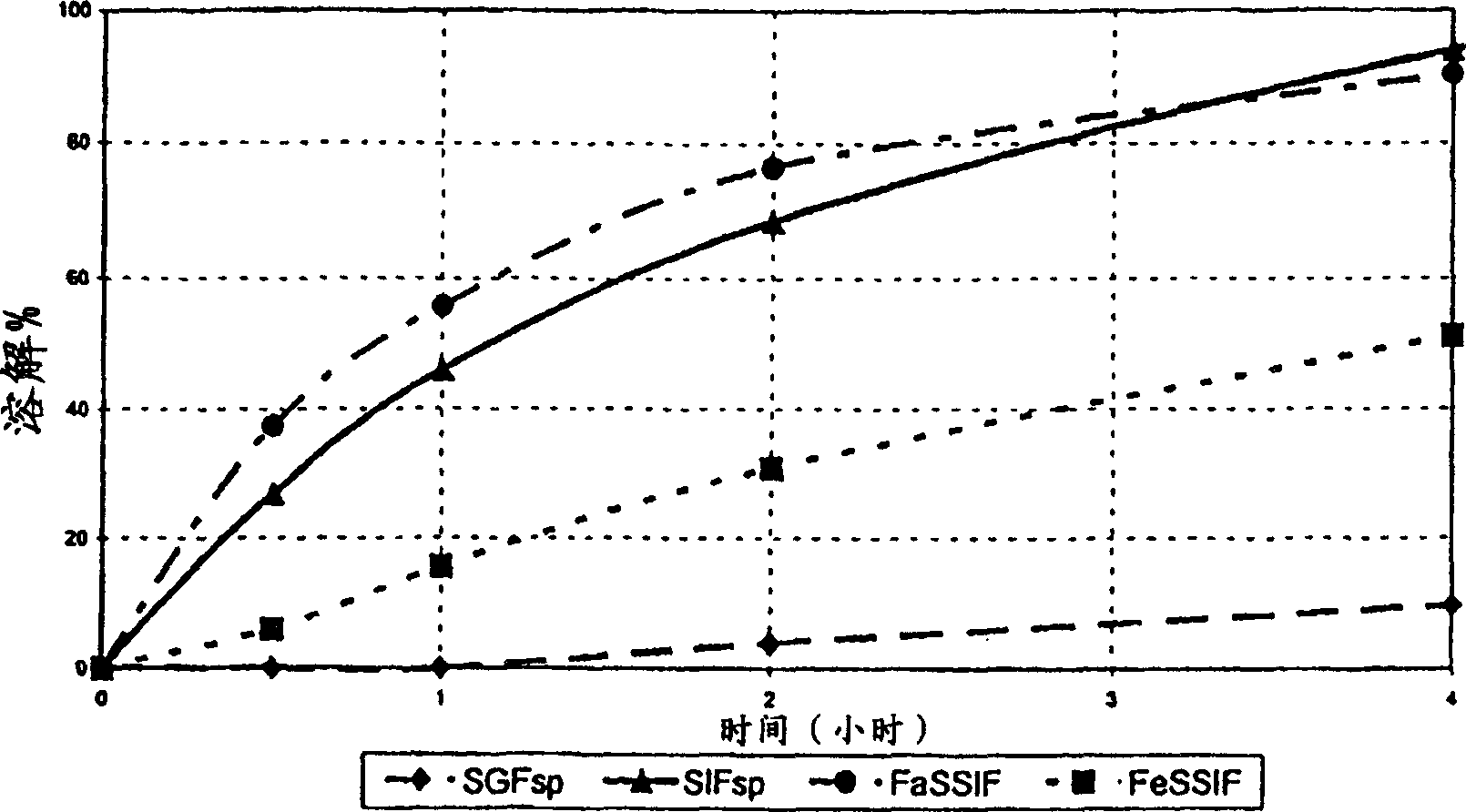
[0082] Three batches of monolithic tablets were prepared by step-blending and direct compression with the following properties:
[0083] (%)
0.4mg
0.5
26.4mg
33.0
26.4mg
33.0
Hydroxypropyl Methyl Cellulose (HPMC)
26.4mg
33.0
0.4mg
0.5
total
80mg
100
[0084] The difference from batch to batch is only the viscosity value of the selected hydroxypropyl methylcellulose:
[0085] Batch A contains METHOCEL K4M CR PREMIUM
[0086] Batch B contains METHOCEL K15M CR PREMIUM
[0087] Batch C containing METHOCEL K100M CR PREMIUM
[0088] b) Operation method
[0089] Tamsulosin hydrochloride was blended (15 minutes) with anhydrous lactose in a 1:9 ratio (10% active substance), ground (15 seconds) and then blended again (5 minutes). This pre-blend was then mixed with the remainde...
Embodiment 2
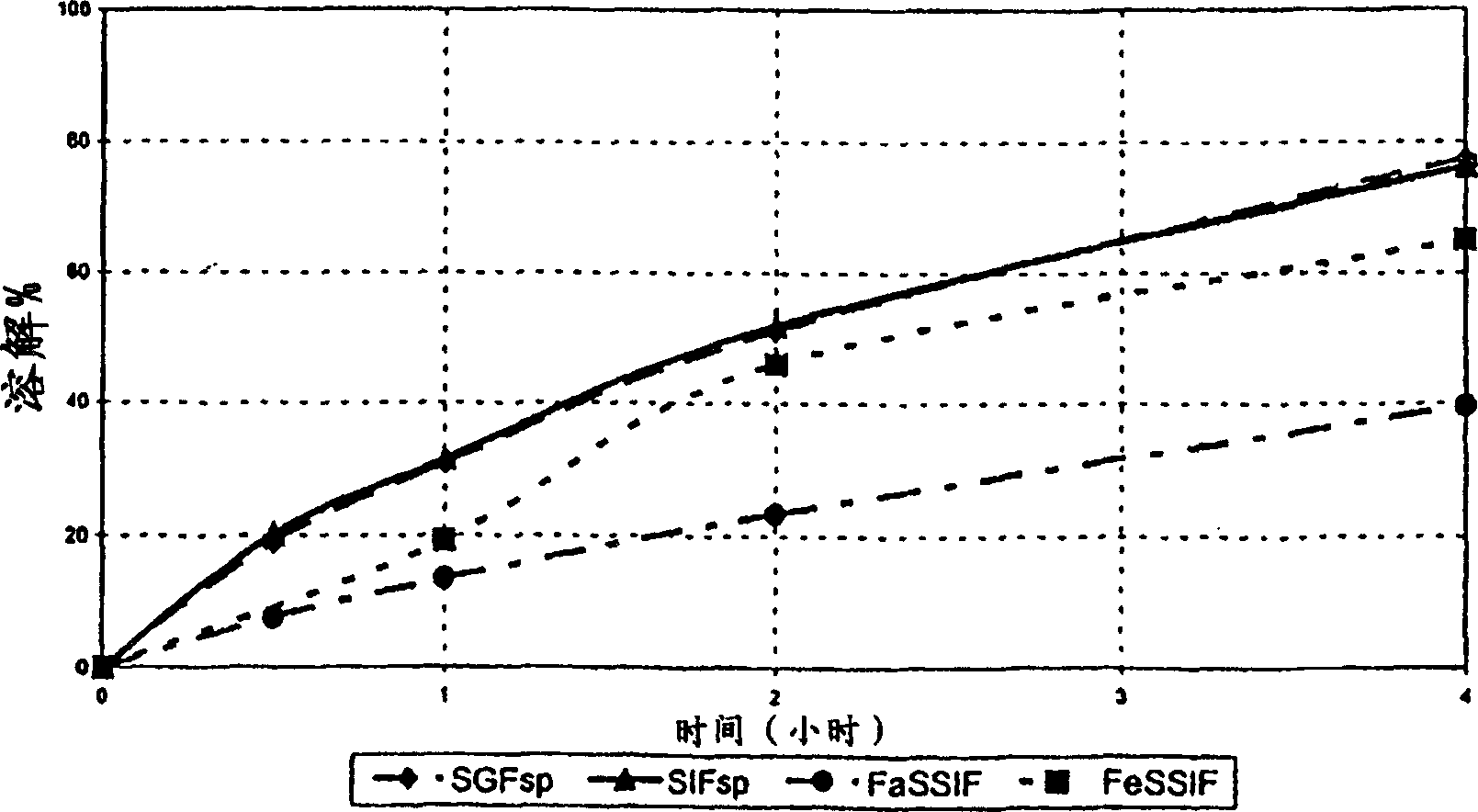
[0098] Three batches of monolithic tablets were prepared by step-blending and direct compression with the following properties:
[0099] D
E
F
0.4mg
0.4mg
0.4mg
35.2mg
30.8mg
22.0 mg
35.2mg
30.8mg
22.0 mg
Hydroxypropyl Methyl Cellulose (HPMC)
8.8mg
17.6mg
35.2mg
0.4mg
0.4mg
0.4mg
total
80mg
80mg
80mg
[0100] The difference from batch to batch is only the concentration of hydroxypropyl methylcellulose used:
[0101] Lot D contains 11% METHOCEL K100M CR PREMIUM
[0102] Lot E contains 22% METHOCEL K100M CR PREMIUM
[0103] Batch F contains 44% METHOCEL K100M CR PREMIUM
[0104] b) Operation method
[0105] Tamsulosin hydrochloride was blended (15 minutes) with anhydrous lactose in a 1:9 ratio (10% active substance), ground (15 seconds) and then...
Embodiment 3
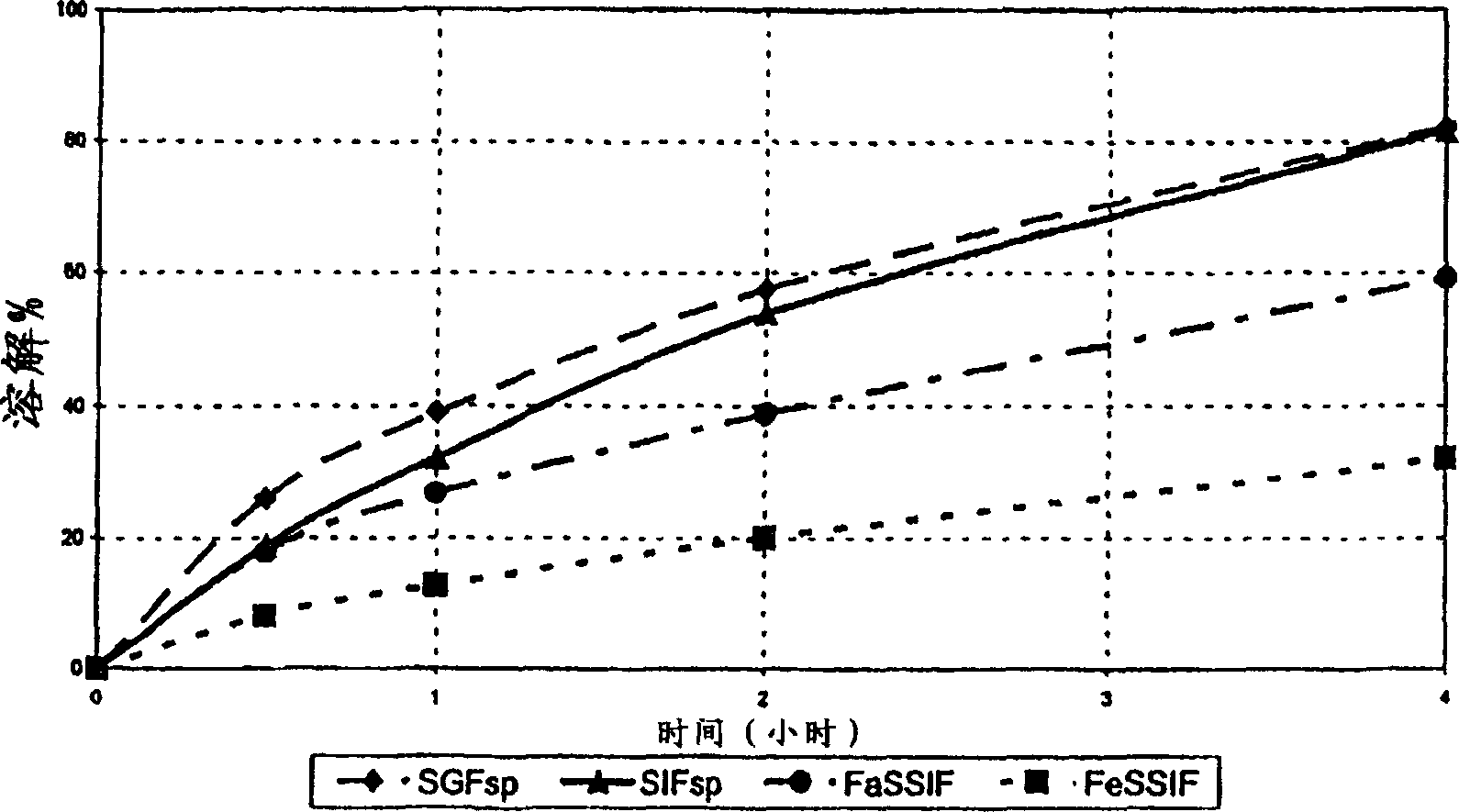
[0111] Two batches of monolithic tablets were prepared by step-blending and direct compression with the following properties:
[0112] a) Tablet composition
[0113] (%)
0.4mg
0.5
anhydrous lactose
25.6mg
32.0
25.6mg
32.0
Hydroxypropyl Methyl Cellulose (HPMC)
28.0mg
35.0
0.4mg
0.5
total
80mg
100
[0114] The differences between the two batches are mainly in scale-up, mixing time and physical parameters.
[0115] G batch enlarged to 20000 units
[0116] Batch H enlarged to 40000 units
[0117] b) Operation method
[0118] Tamsulosin hydrochloride was blended (Turbula; 15 minutes) with anhydrous lactose in a 1:9 ratio (10% active substance), ground (IKA; 30 seconds) and then blended again (Turbula; 5 minutes). This pre-blend was then mixed with the remainder of the lactose, dical...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com