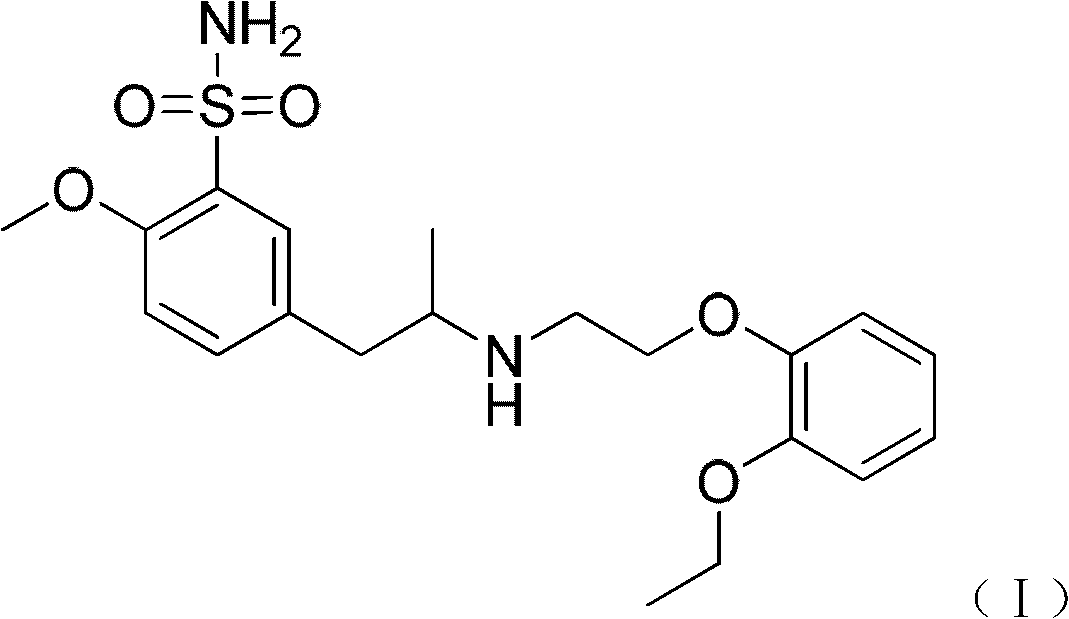
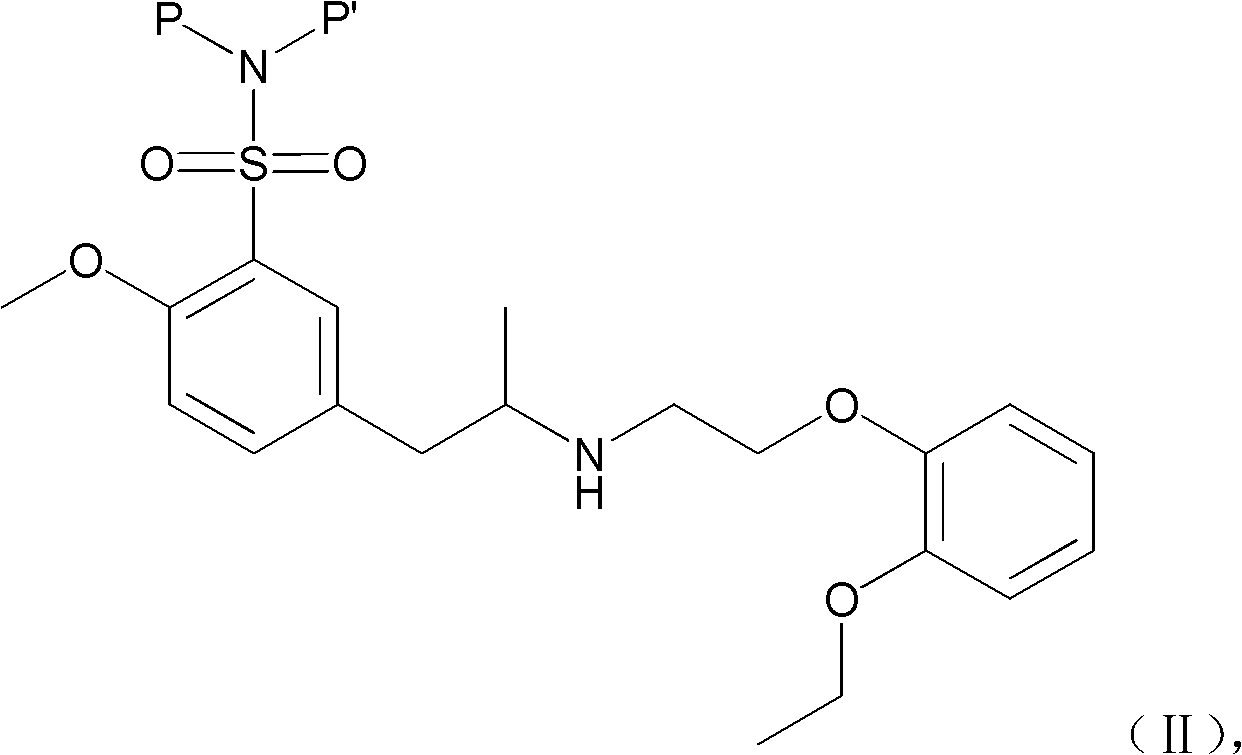
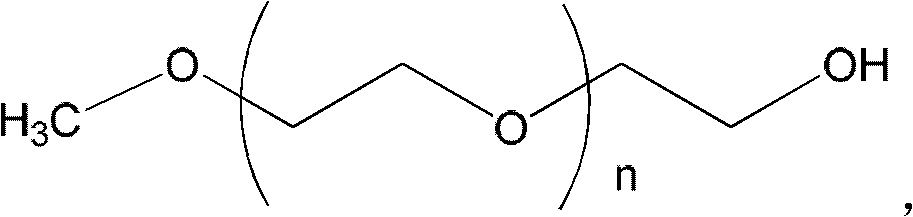
Combination of polyethylene glycol and tamsulosin and pharmaceutical compound comprising same
A technology of polyethylene glycol and octyl conjugates, which is applied in the field of small molecule oligoethylene glycol and tamsulosin conjugates and their pharmaceutical compositions, which can solve problems such as neuropsychiatric adverse reactions and improve drug quality. Absorption, enhancing curative effect, and avoiding toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of Conjugate of Methoxytetraethylene Glycol and Tamsulosin (TSLX41)
[0053]
[0054] Add 9.07g p-toluenesulfonyl chloride and 32mL pyridine into a 250mL three-necked flask, and cool to 0°C. Mix 8.26gmPEG(n=4)-OH and 16mL of pyridine, dropwise into a three-necked flask, and finish dropping in two hours, controlling the temperature at 0-10°C. The reaction was continued to stir at this temperature for two hours. TLC monitored the completion of the reaction. Add 130mL of cold water and 48mL of concentrated hydrochloric acid to the reaction solution, stir slowly for half an hour, transfer the reaction solution into a 500mL separatory funnel, add ethyl acetate and extract twice (200mL+100mL). The organic layers were combined, washed with water until neutral, and dried over anhydrous sodium sulfate for two hours. The solvent was evaporated on a rotary evaporator. A total of 11.34 g of viscous liquid was obtained, which was directly used in the next reaction...
Embodiment 2
[0058] Preparation of Conjugate of Dimethoxypentaethylene Glycol and Tamsulosin (TSLX52)
[0059]
[0060] Add 356 mg of tamsulosin hydrochloride and 664 mg of potassium carbonate into a 100 mL three-necked flask containing 20 mL of acetonitrile, and stir at room temperature for 2 hours. A 10 mL acetonitrile solution dissolved with 759 mg mPEG(n=5)Br was added into the above reaction flask, and reacted under reflux overnight. TLC monitored the completion of the reaction. Column separation gave 258 mg of a colorless liquid with a yield of 36.8%. m / z[MH] + 877.8. 1 H-NMR (CDCl 3 ): 1.10(d, J=6.2Hz, 3H), 1.39-1.44(t, J=6.9Hz, 3H), 2.60(m, 1H), 2.90(m, 1H), 3.05-3.13(m, 4H) , 3.37(s, 6H), 3.45-3.55(m, 20H), 3.60-3.65(m, 20H), 3.90(s, 3H), 4.07(m, 2H), 4.17(t, J=5.2Hz, 2H ), 6.90 (m, 5H), 7.37 (dd, J=8.5Hz, J=2.2Hz, 1H), 7.74 (d, J=2.2Hz, 1H).
Embodiment 3
[0062] Preparation of Double-ended Hexaethylene Glycol and Tamsulosin Conjugate (TSLXP62)
[0063]
[0064] Add 22.9g of p-toluenesulfonyl chloride and 80mL of pyridine into a 500mL three-necked flask, and cool to 0°C. Mix 14.1g of HO-PEG(n=6)-OH with 40mL of pyridine and add it dropwise into a three-necked flask. The dropwise is completed in two hours, and the temperature is controlled at 0-10°C. The reaction was continued to stir at this temperature for two hours. TLC monitored the completion of the reaction. Add 300mL cold water and 120mL concentrated hydrochloric acid to the reaction solution, stir slowly for half an hour, transfer the reaction solution into a 1000mL separatory funnel, add ethyl acetate and extract twice (300mL+200mL). The organic layers were combined, washed with water until neutral, and dried over anhydrous sodium sulfate for two hours. The solvent was evaporated on a rotary evaporator. A total of 28.7 g of viscous liquid was obtained, which was d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com