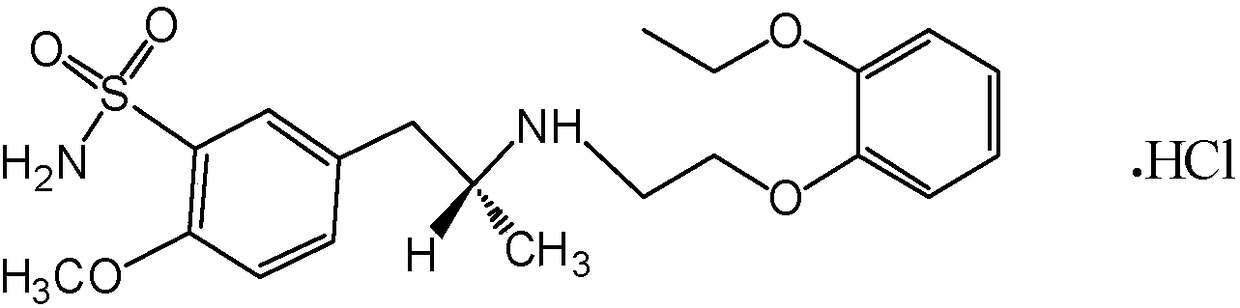
Tamsulosin orally disintegrating tablet composition with slow release performance
A technology for orally disintegrating tablets and tablets, applied to the preparation of tamsulosin hydrochloride orally disintegrating sustained-release tablet compositions with excellent pharmaceutical effects, and in the field of preparing tamsulosin medicaments, which can solve the problem of increasing the inoperability of the process And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Example 1: Preparation of Tamsulosin Hydrochloride Orally Disintegrating Tablets
[0230] (1) Dissolve 80 g of tamsulosin hydrochloride and 80 g of hydroxypropyl methylcellulose in 2000 g of purified water. Microcrystalline cellulose microparticles (Celphere102, Asahi Kasei, average particle diameter is about 120 μm, more than 95% particle diameter is in the range of 50-150 μm) 4000g is put into fluidized bed granulator, wraps with this solution with lateral spraying method Clothing to obtain tamsulosin hydrochloride particles.
[0231] The fluidized bed granulator above and in the context of the present invention is the WBF-60 fluidized bed granulator produced by Chongqing Yingge Granulation Coating Technology Co., Ltd. When using this fluidized bed granulator for granulation and / or coating, according to different materials, the spray speed can usually be controlled in the range of 10-120g / min, and the spray air pressure can usually be controlled in the range of 0.5-...
Embodiment 2
[0282] Example 2: Preparation of Tamsulosin Hydrochloride Orally Disintegrating Tablets
[0283] (1) Dissolve 80 g of tamsulosin hydrochloride and 80 g of hydroxypropyl methylcellulose in 2000 g of purified water. Put microcrystalline cellulose microparticles (Celphere102, Asahi Kasei, average particle diameter is about 160 μm, more than 95% particle diameter is in the scope of 100-250 μm) 4000g into fluidized bed granulator, use this solution to pack Clothing to obtain tamsulosin hydrochloride particles.
[0284] (2) Next, add 550 g of ethyl cellulose (ground and pass through a 200-mesh sieve) and 160 g of hydroxypropyl methylcellulose into 18,000 g of water, suspend the ethyl cellulose strongly and dissolve HPMC to obtain slow Release the coating suspension. Put 4000 g of tamsulosin hydrochloride particles into a fluidized bed granulator, and coat with the coating liquid by a side spraying method to obtain sustained-release pellets.
[0285] (3) Put 4000g of the slow-re...
Embodiment 3
[0298] Example 3: Preparation of Tamsulosin Hydrochloride Orally Disintegrating Tablets
[0299] (1) Dissolve 80 g of tamsulosin hydrochloride and 80 g of hydroxypropyl methylcellulose in 2000 g of purified water. Microcrystalline cellulose particles (Celphere102, Asahi Kasei, average particle diameter is about 80 μm, more than 95% of the particle diameter is in the range of 50-120 μm) 4000g is put into a fluidized bed granulator, and the solution is packaged with the side spray method. Clothing to obtain tamsulosin hydrochloride particles.
[0300] (2) Next, add 600 g of ethyl cellulose (ground and pass through a 200-mesh sieve) and 180 g of hydroxypropyl methylcellulose into 20,000 g of water, suspend the ethyl cellulose strongly and dissolve HPMC to obtain slow Release the coating suspension. Put 4000 g of tamsulosin hydrochloride particles into a fluidized bed granulator, and coat with the coating liquid by a side spraying method to obtain sustained-release pellets.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Sheet weight | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com