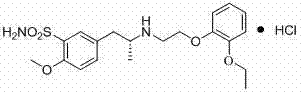
Synthesis method of tamsulosin hydrochloride
A technology of tamsulosin hydrochloride and a synthesis method, which is applied in the field of synthesis of tamsulosin hydrochloride, can solve the problems of complex synthesis process of tamsulosin hydrochloride, high cost, impure product and the like, and achieves good product purity and synthesis. Convenience and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
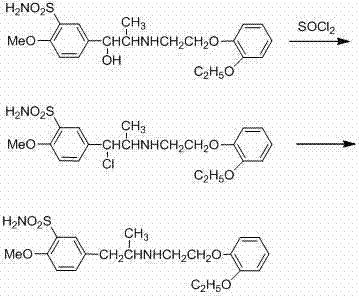
[0052] A kind of synthetic method of tamsulosin hydrochloride, described method is carried out according to the following steps:
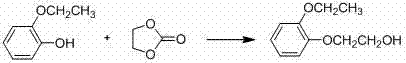
[0053] (1) Condensation reaction: In a 1000ml reaction bottle, add RMBS (39.3g, 98%, the amount of substance is 0.245mol), EPEB 0.245mol, potassium hydroxide 0.3675mol and acetonitrile, wherein the amount of acetonitrile is the total amount of RMBS and EPEB 3 times the mass, heated up to 40°C, reacted for 15h, then lowered the temperature, filtered, the filtrate was distilled under reduced pressure to recover acetonitrile, and obtained condensate intermediate 53.2g, see Table 1, the purity of the condensate intermediate was 94.8%, and the yield was 93.5 % (Based on RMBS quality), the condensate intermediate is directly subjected to the next hydrogenation reaction without purification;
[0054] (2) Hydrogenation reaction: Take 50g of the condensate intermediate, add ethanol (150g) which is 3 times the mass of the condensate intermediate in a 1000ml ...
Embodiment 2
[0056] The basic operation steps of embodiment 2, embodiment 3, embodiment 4 and embodiment 5 are the same as embodiment 1, and the specific reaction conditions and results are shown in table 1.
[0057] Shown by table 1, synthetic method reaction condition of the present invention is gentle. During the reaction process, there are few by-products, the yield and purity of the intermediate product are high, and the obtained final product tamsulosin hydrochloride has good product purity and high yield.
[0058] Table 1 Concrete reaction conditions and results of embodiment 1 to embodiment 5
[0059]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com