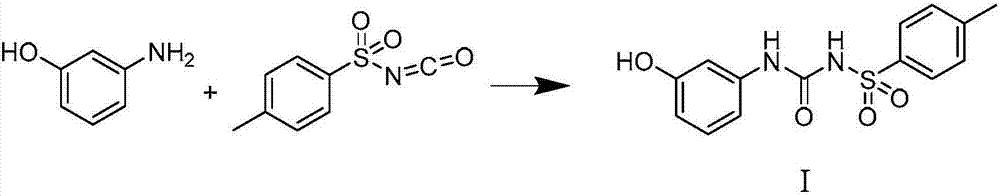
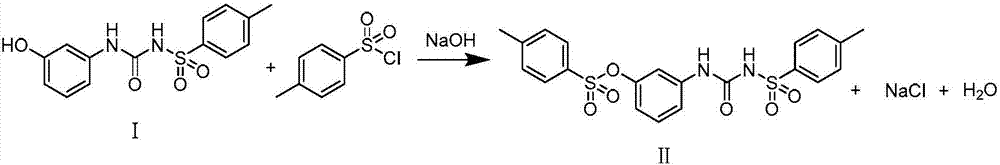
Synthesis method of thermosensitive sensitizer N-p-tolylsulfonyl-N'-(3-p-tolylsulfonyl oxyphenyl) urea
A technology of toluenesulfonyloxyphenyl and p-toluenesulfonyl, which is applied in the field of synthesis of heat-sensitive sensitizers, can solve the problems of low total yield, and achieve the effects of simple operation, suitability for industrial production, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add m-aminophenol (10.3g, 0.0945mol) and p-toluenesulfonyl isocyanate (20.45g, 0.104mol) to acetonitrile (90mL) successively, stir at room temperature overnight, then add 50mL of water and (10.7g, 0.126mol) 47% Sodium hydroxide aqueous solution, then add p-toluenesulfonyl chloride (19.86g, 0.104mol), after addition, react at 40°C for 2 hours, cool down to room temperature, neutralize with hydrochloric acid to precipitate a white solid, filter, wash with water, and dry to obtain 42.6g of white solid N-p-toluenesulfonyl-N'-(3-p-toluenesulfonyloxyphenyl) urea, the yield is 98%, and the melting point is 156-159°C.
[0025] The characterization result of product N-toluenesulfonyl-N'-(3-toluenesulfonyloxyphenyl) urea is as follows:
[0026] 1 H NMR (500MHz, DMSO-d 6 )δ=10.81(s,1H),9.13(s,1H),7.82-7.84(d,2H),7.70-7.71(d,2H),7.42-7.44(d,4H),7.17-7.25(m, 3H), 6.57-6.59(d, 1H), 2.40(s, 6H) ppm.ESI-MS: (m / z, %)=461[M+H] + .
Embodiment 2
[0028] Add m-aminophenol (9.32g, 0.0855mol) and p-toluenesulfonyl isocyanate (20.45g, 0.104mol) to acetonitrile (100mL) successively, stir at 40°C for 4h, then add 50mL of water and (15.7g, 0.196mol) 50% sodium hydroxide aqueous solution, then add p-toluenesulfonyl chloride (15.89g, 0.0832mol), after addition, react at 60°C for 4.5 hours, cool down to room temperature, neutralize with hydrochloric acid to precipitate a white solid, filter, wash with water, and dry to obtain a white solid N-p-toluenesulfonyl-N'-(3-p-toluenesulfonyloxyphenyl) urea, the yield is 97%, and the melting point is 155-158°C.
Embodiment 3
[0030] Add m-aminophenol (13.60g, 0.125mol) and p-toluenesulfonyl isocyanate (20.45g, 0.104mol) to acetonitrile (150mL) successively, stir at room temperature overnight, then add 50mL of water and (12.19g, 0.0914mol) 30% Sodium hydroxide aqueous solution, then add p-toluenesulfonyl chloride (23.30g, 0.122mol), after addition, react at 90°C for 1 hour, cool down to room temperature, neutralize with hydrochloric acid to precipitate a white solid, filter, wash with water, and dry to obtain a white solid N- p-toluenesulfonyl-N'-(3-p-toluenesulfonyloxyphenyl) urea, the yield is 98%, and the melting point is 154-158°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com