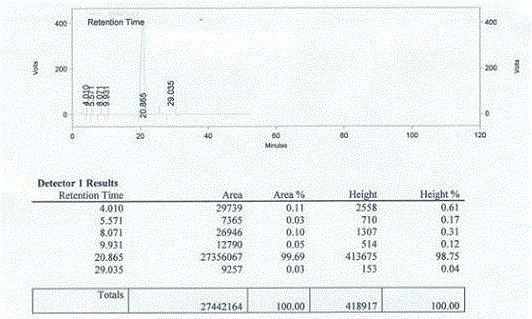
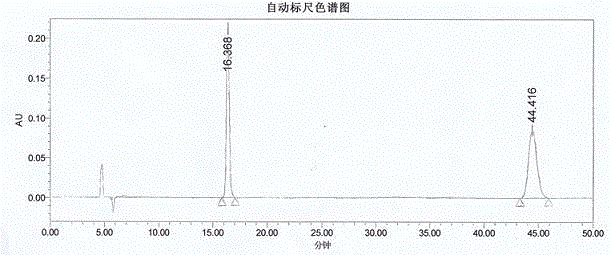
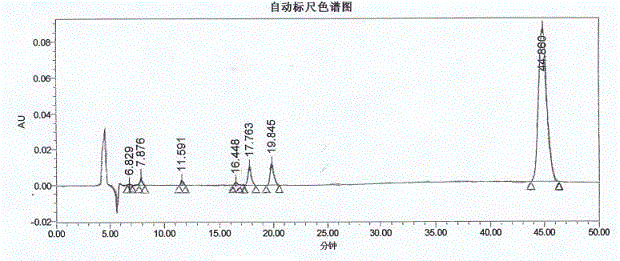
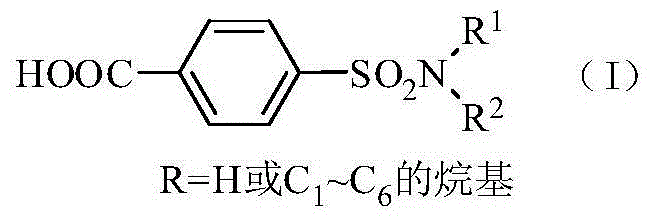
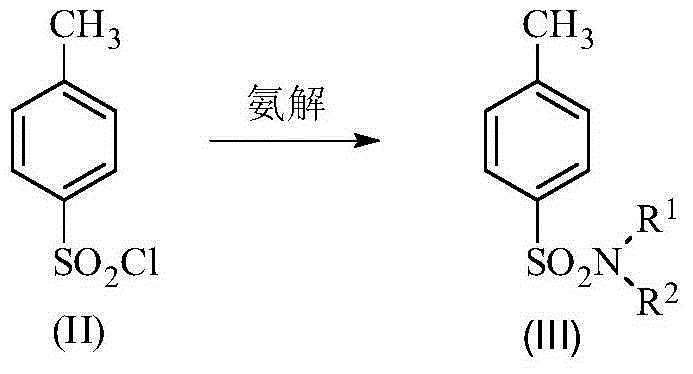
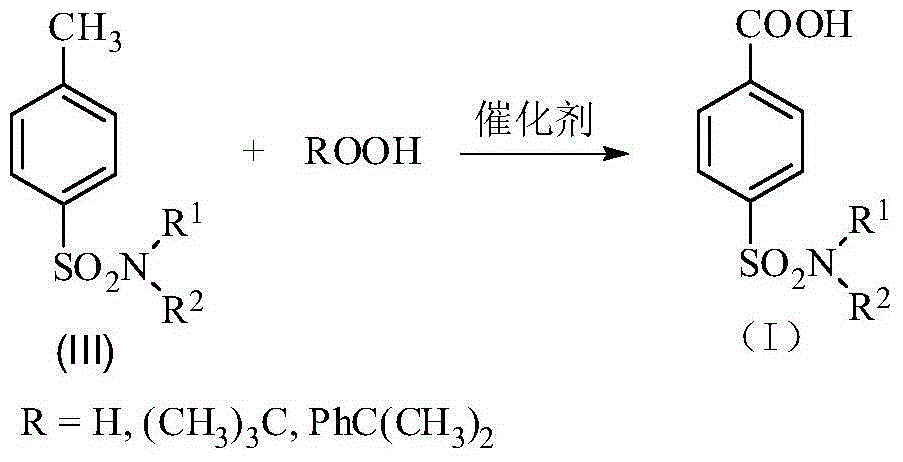
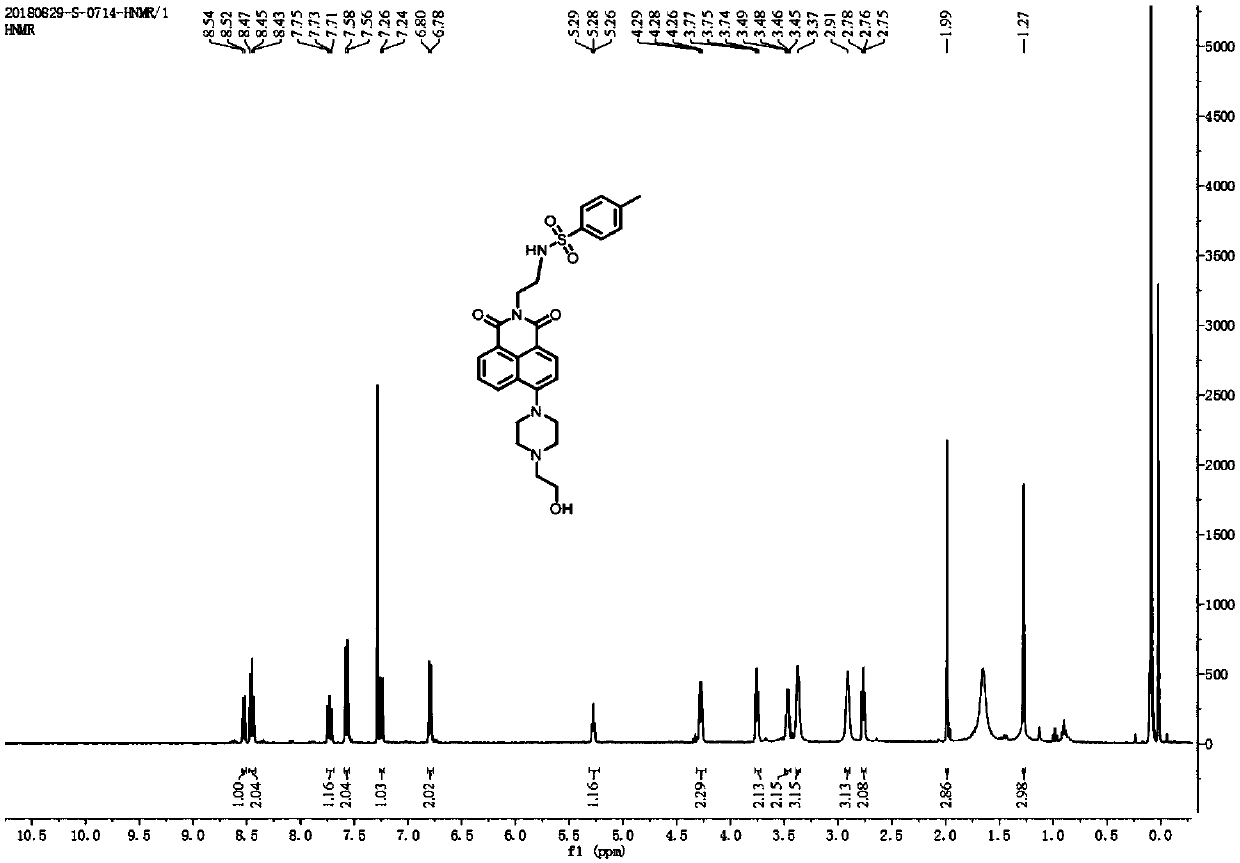
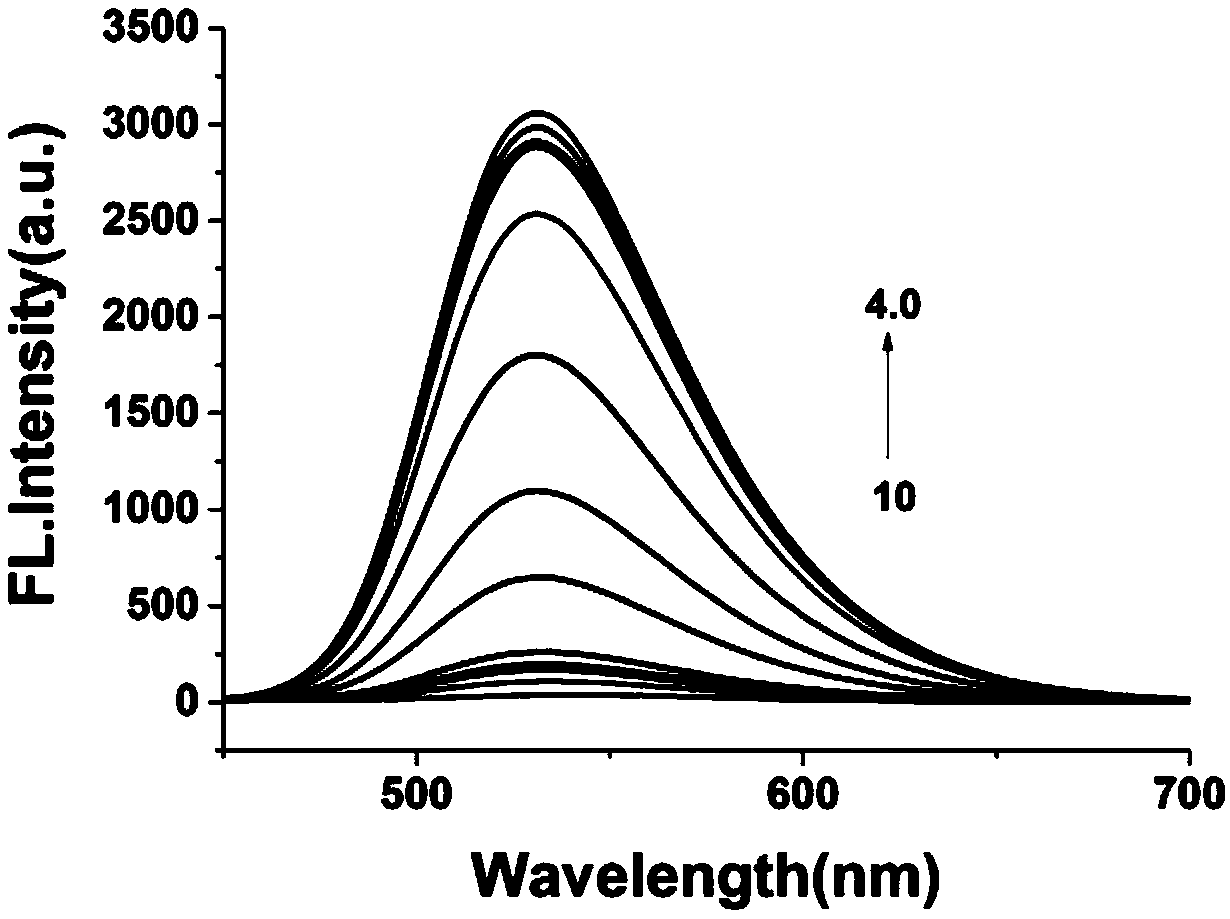
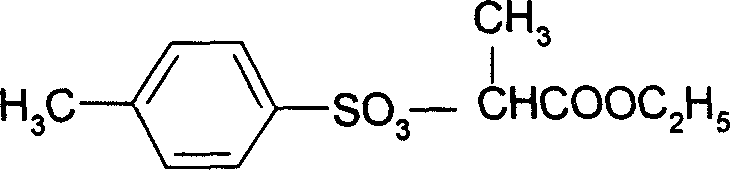Patents
Literature
434 results about "P-toluenesulfonyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
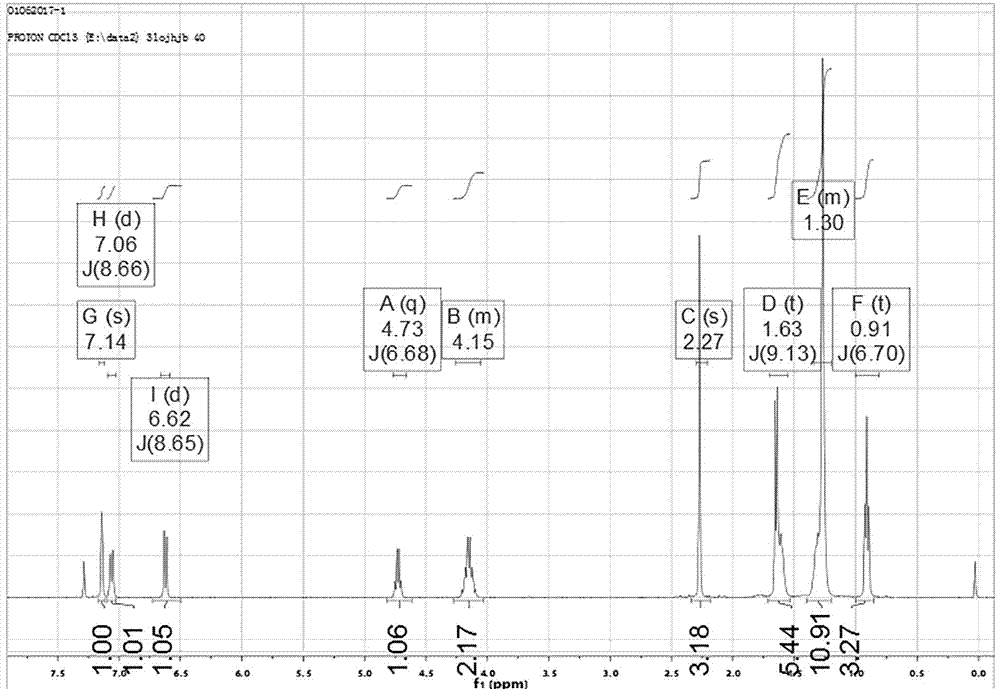
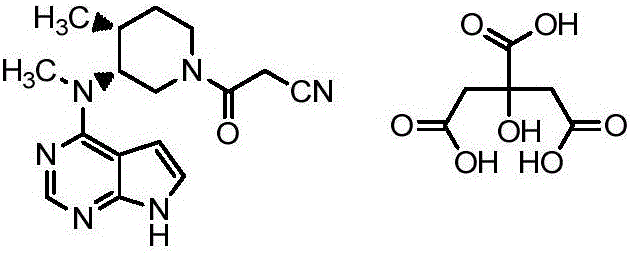
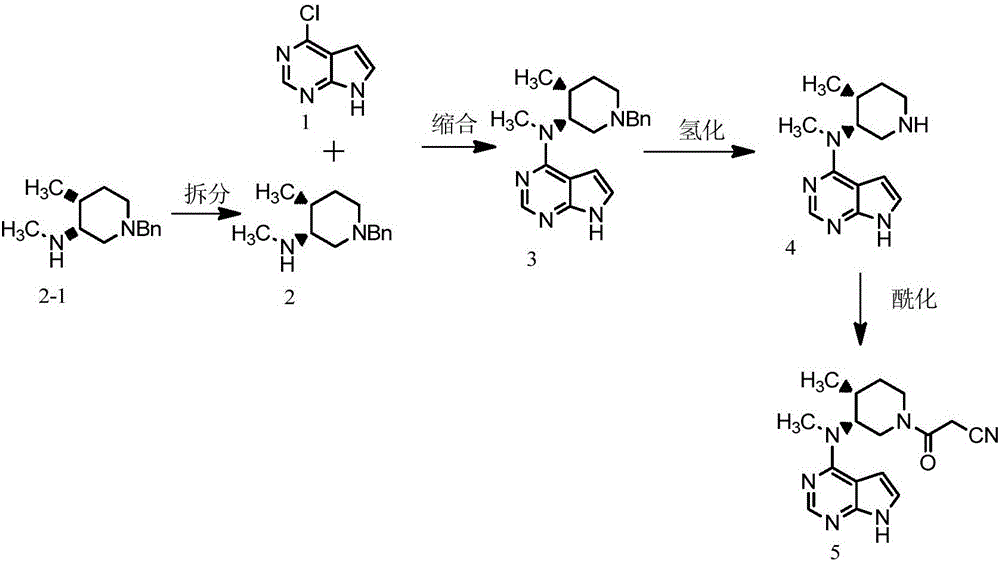
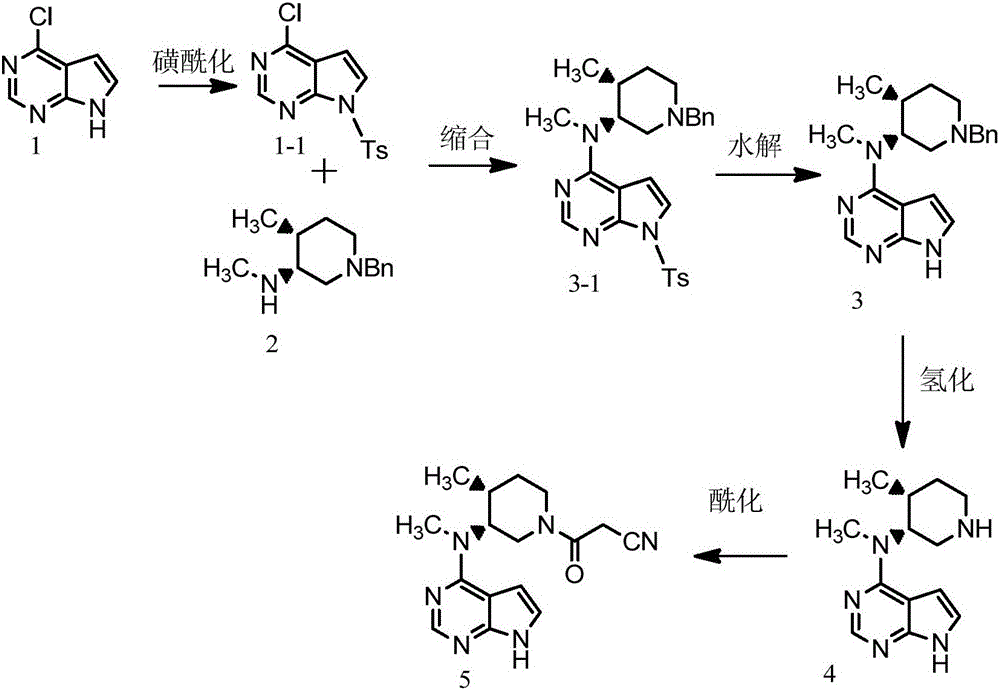
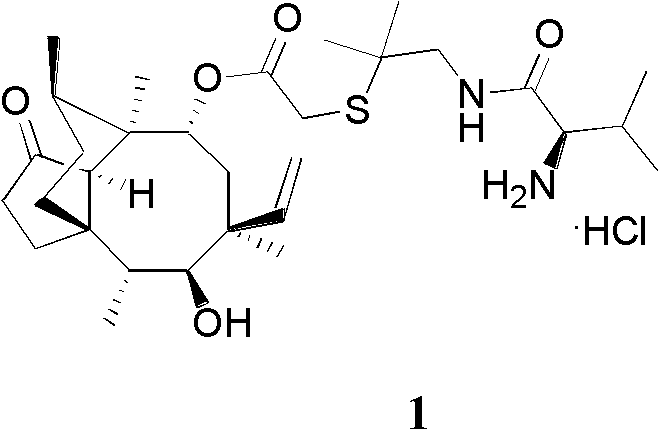
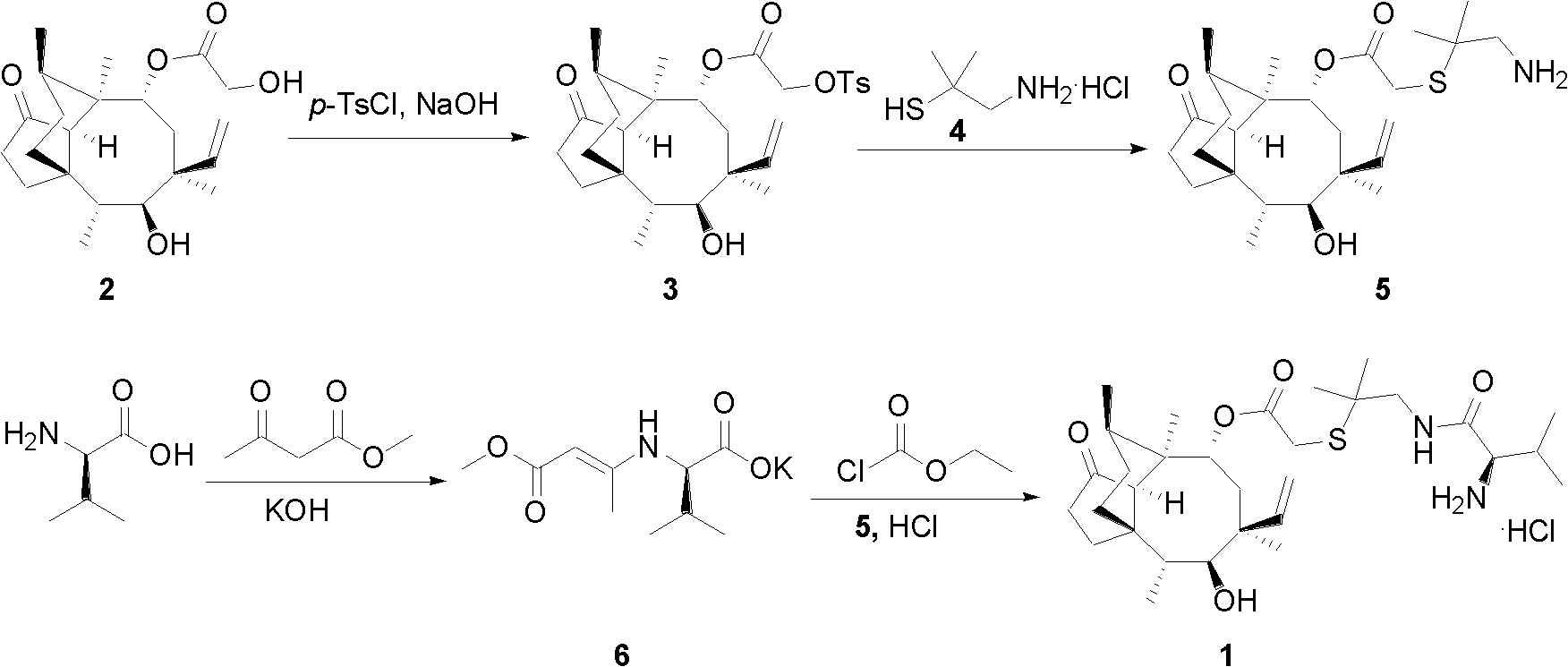
Preparation method of tofacitinib citrate
ActiveCN105884781AEasy to purifyGood stereoselectivityOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsCompound (substance)Benzyl group
The invention belongs to the field of medicine and chemical engineering and particularly relates to a preparation method of tofacitinib citrate. The method comprises steps as follows: 1-benzyl-4-methyl-2,6-dihydro-3-piperidone taken as a starting material is subjected to an asymmetric reduction reaction, 1-benzyl-4-methyl-3-piperidone is obtained, and (3R,4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochloride is produced under the action of a chiral catalyst; (3R,4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochlorid and a paratoluensulfonyl chloride protection product 4-chloro-7-(methyl-4-benzenesulfonyl) pyrrolo[2,3-d]pyrimidine of 4-chloropyrrolo[2,3-d]pyrimidine are subjected to a condensation reaction, [(3R,4R)]-1-benzyl-4-methyl-piperidine-3-yl]-methyl-(7H-pyrrolo[2,3-d]pyrimidine-4-yl)-amine is obtained through deprotection, and tofacitinib citrate is obtained through debenzylation protection, an acylation reaction and citric acid salifying. The process route is short, the process cycle is short, chiral synthesis is performed by means of a catalyst, the product purity is improved, the cost is reduced, the yield is high, and the operation is simple and convenient.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
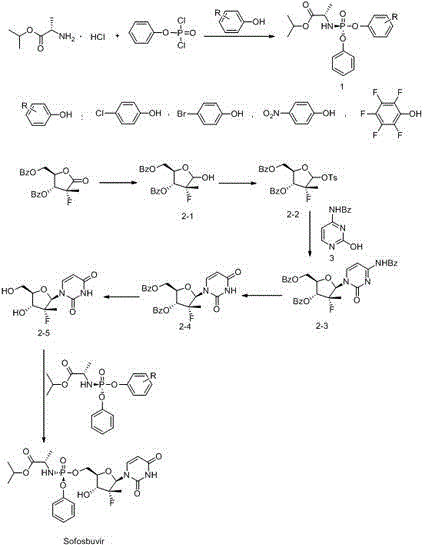
Preparation method of ribofuranose phosphate derivative
ActiveCN104610404APurity is easy to controlHigh yield of docking reactionSugar derivativesSugar derivatives preparationPhosphateGrignard reagent
The invention discloses a preparation method of a ribofuranose phosphate derivative. The preparation method comprises preparation steps as follows: L-alanine isopropyl ester hydrochloride, phenol dichlorophosphate and substituted phenol are taken as starting materials and have a docking reaction under the action of alkali; (2R)-2-deoxy-2-difluoro-2-methyl-D-erythropentonic acid GAMMA-lactone and 3,5-dibenzoate reduce carbonyl in a dichloromethane or ether solvent into an alcoholic hydroxyl group under the action of a strong reducing agent; an intermediate with a formula 2-1 has a reaction with paratoluensulfonyl chloride under the action of alkali to obtain p-toluenesulfonates; an intermediate with a formula 2-2 and a benzoyl cytosine derivative have a docking reaction under the action of a condensing agent; an intermediate with a formula 2-3 converts cytosine into uracil under the action of organic acid; benzoyl protection for an intermediate with a formula 2-4 is released under the action of an alkaline agent; an intermediate with a formula 2-5 and an intermediate with a formula 1 are docked under the action of a Grignard reagent to obtain Sofosbuvir.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
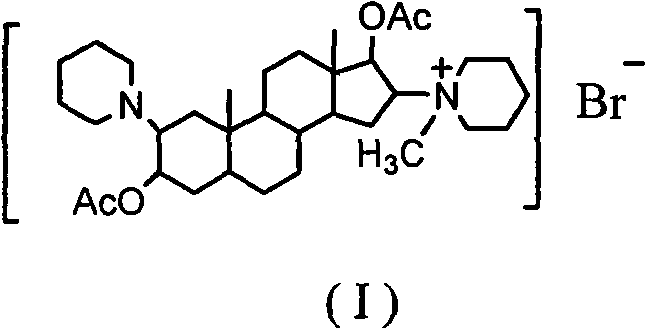
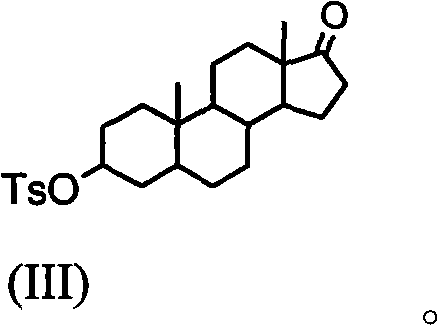
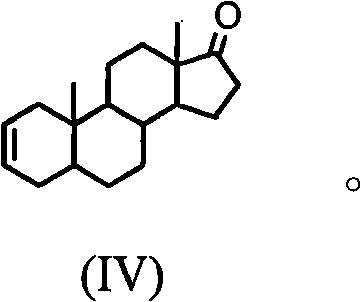
Synthesis process of vecuronium bromide
The invention discloses a synthesis process of vecuronium bromide. The synthesis process comprises the following steps: generating epiandrosterone sulfonyl ester (III) by carrying out esterification reaction between epiandrosterone (II) and paratoluensulfonylchloride; generating 5Alpha-androst-2-alkene-17-ketone (IV) by carrying out elimination and dehydration reaction between the (III) and 2,6-lutidines; generating 17-acetoxyl-5Alpha-androstane-2,16-diene (V) by carrying out enolization and esterification reaction between the (IV) and isopropenyl acetate; generating (2Alpha, 3Alpha, 16Alpha,17Alpha)-diepoxy-17Beta-acetyl-5Alpha-androstane (VI) by epoxy reaction of the (V) under the effect of hydrogen peroxide; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha-hydroxyl-17-ketone (VII) by ring-opening and addition reaction of the (VI) under the effect of hexahydropyridine; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha,17Beta-diol (VIII) by the (VII)under the reduction of potassium borohydride; generating 2Beta, 16Beta-di(1-piperidyl)-3Alpha, 17Beta- acetoxyl-5Alpha-androstane (IX) by carrying out esterification reaction of the (VIII) under the acetylation of acetic anhydride; and generating vecuronium bromide (I) by carrying out quaternary ammonium salt reaction between the (IX) and bromomethane. The invention has the advantages of low cost,less pollution and high yield.
Owner:XUZHOU NORMAL UNIVERSITY
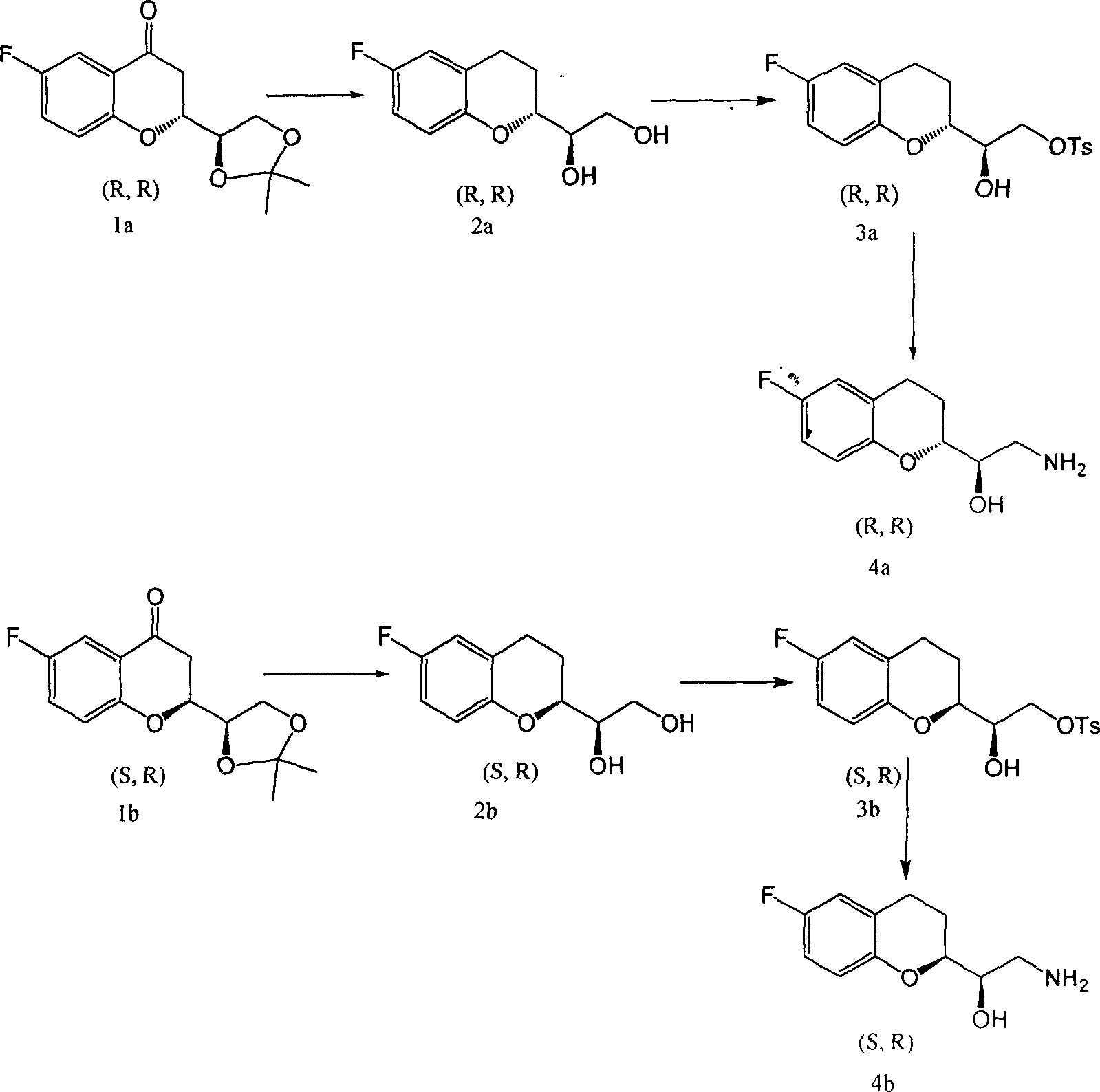
Synthetic process of chiral 2-amido-1-(6-fluorine-3,4-dihydrobenzopyranyl) alCohol
This invention is attributed to the field of organic chemistry and specifically relates to the method to synthesize drug intermediates (R)-2-amino-1-((R)-6-fluoro-3, 4-dihydrochromeno) ethanol and (R)-2-amino-1-((S)-6-fluoro-3, 4-dihydrochromeno) ethanol. The method is that, drug intermediates (2R)-2-[(1R)-4, 4-dimethyl-3, 5-dioxocyclopentyl]-6-fluoro-4-chromanone and (2S)-2-[(1R)-4, 4-dimethyl-3, 5-dioxocyclopentyl]-6-fluoro-4-chromanone are adopted as raw materials and reduced by Clemmensens method with the products reacting with p-toluenesulfonyl chloride in pyridine. The consequent products are dissolved in solvent and dry ammonia is introduced for heating reflux. The total yield can be as much as 32%.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
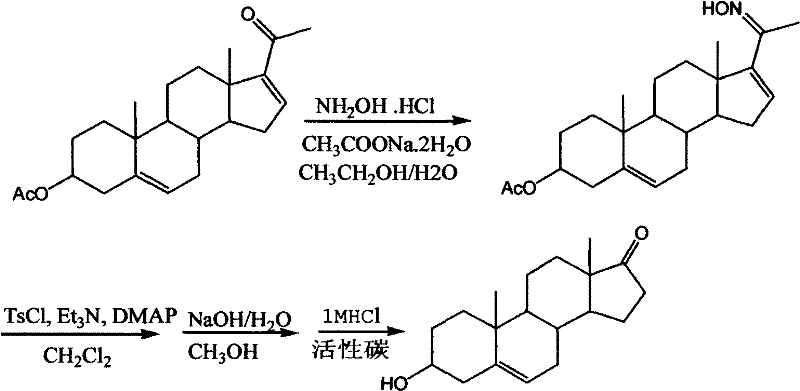
Synthesis method for dehydroepiandrosterone
InactiveCN102212099AOvercoming reactivityOvercoming processingSteroidsBeckmann rearrangementSodium acetate
The invention belongs to a novel synthesis method for dehydroepiandrosterone, which comprises the following steps: oximation of 16-dehydropregnenolone acetate, Beckmann rearrangement, hydrolysis and refining to obtain a product. The synthesis method is characterized by carrying out oximation of ketone by using sodium acetate as a base and water and ethanol as solvent, carrying out Beckmann rearrangement, hydrolysis and one-pot refining reaction, reacting 16-dehydropregnenolone acetate oxime with p-toluenesulfonamide chloride, benzenesulfonyl chloride, triethylamine or N,N-dimethyl-pyridine (DMAP) in a dichloromethane solution, concentrating the solvent after reaction, adding methanol and a sodium hydroxide solution for refluxing hydrolysis, cooling and regulating the pH value to 7-8, adding activated carbon, then refluxing for 30-60 minutes, filtering, concentrating, crystallizing, and centrifuging to obtain the product. The synthesis method provided by the invention is simple to operate, and has characteristics of mild reaction conditions, high yield, low environmental pollution and the like.
Owner:HUNAN KEREY BIOTECH
Preparation method and application of modified cyclodextrin polymer hydrogel
ActiveCN108276589APromote degradationReduce manufacturing costOther chemical processesWater contaminantsThioureaPhenol
The invention relates to the technical field of sewage treatment, provides a preparation method and application of cyclodextrin polymer hydrogel, and aims at solving the problems that the conventionalhydrogel is capable of treating single pollutants, and is low in efficiency and small in application range. The preparation method is characterized in that beta-cyclodextrin is used as the raw material and is subjected to selective sulfonylation through toluene sulfochloride to obtain toluenesulfonyl-beta-cyclodextrin; then the toluenesulfonyl-beta-cyclodextrin is subjected to thiourea substituting to obtain hydrosulphonyl grafted beta-cyclodextrin; finally, the hydrosulphonyl grafted beta-cyclodextrin is processed according to a suspension emulsion polymerizing method to obtain the novel two-functional polymer hydrogel. The hydrogel prepared according to the method is applicable to a complex sewage system integrating phenol and heavy metal ions. The hydrogel is capable of efficiently synchronously adsorbing two pollutants including phenol and the heavy metal ions, and is wide in application range and high in adsorption efficiency.
Owner:ZHEJIANG FORESTRY UNIVERSITY
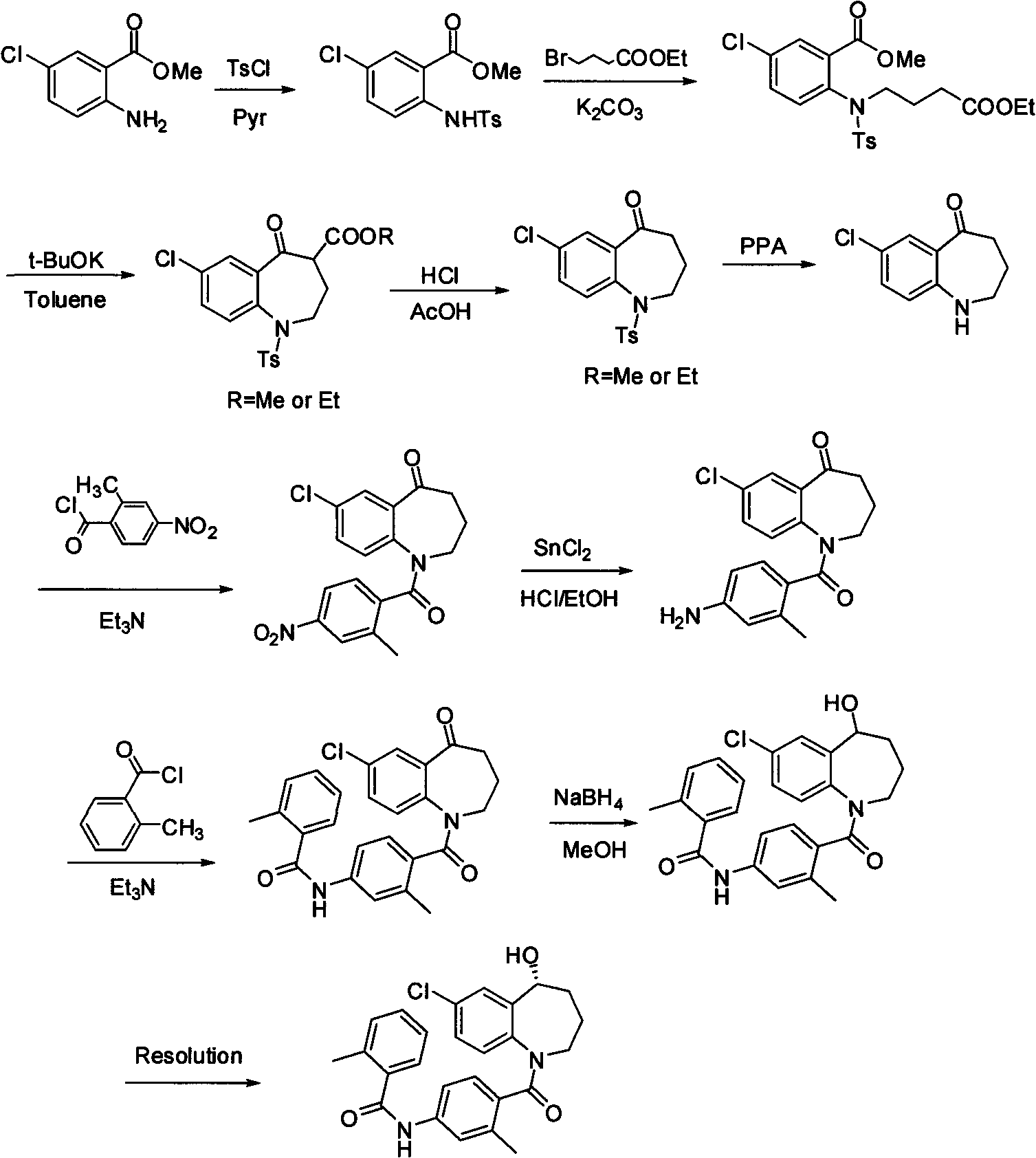
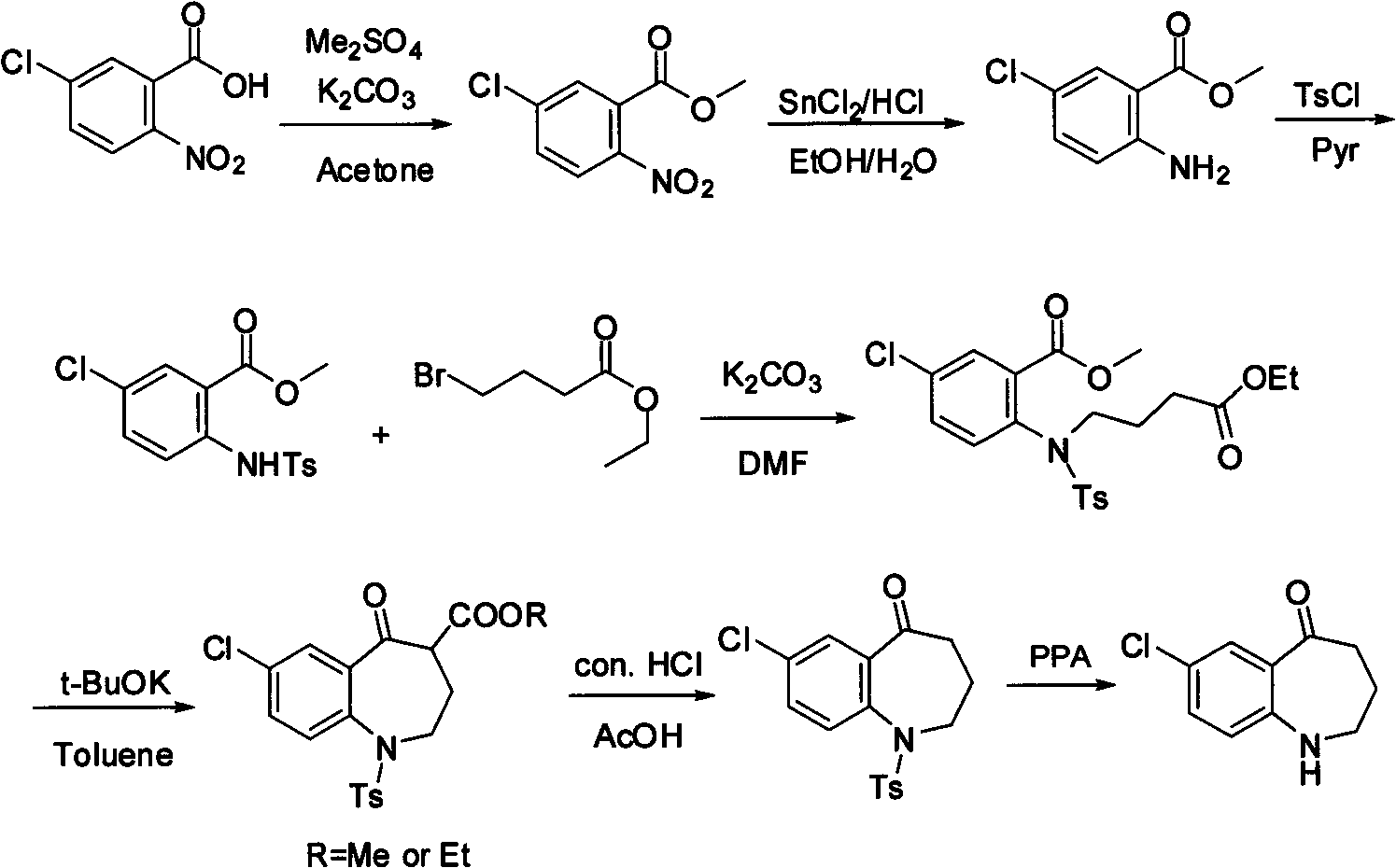
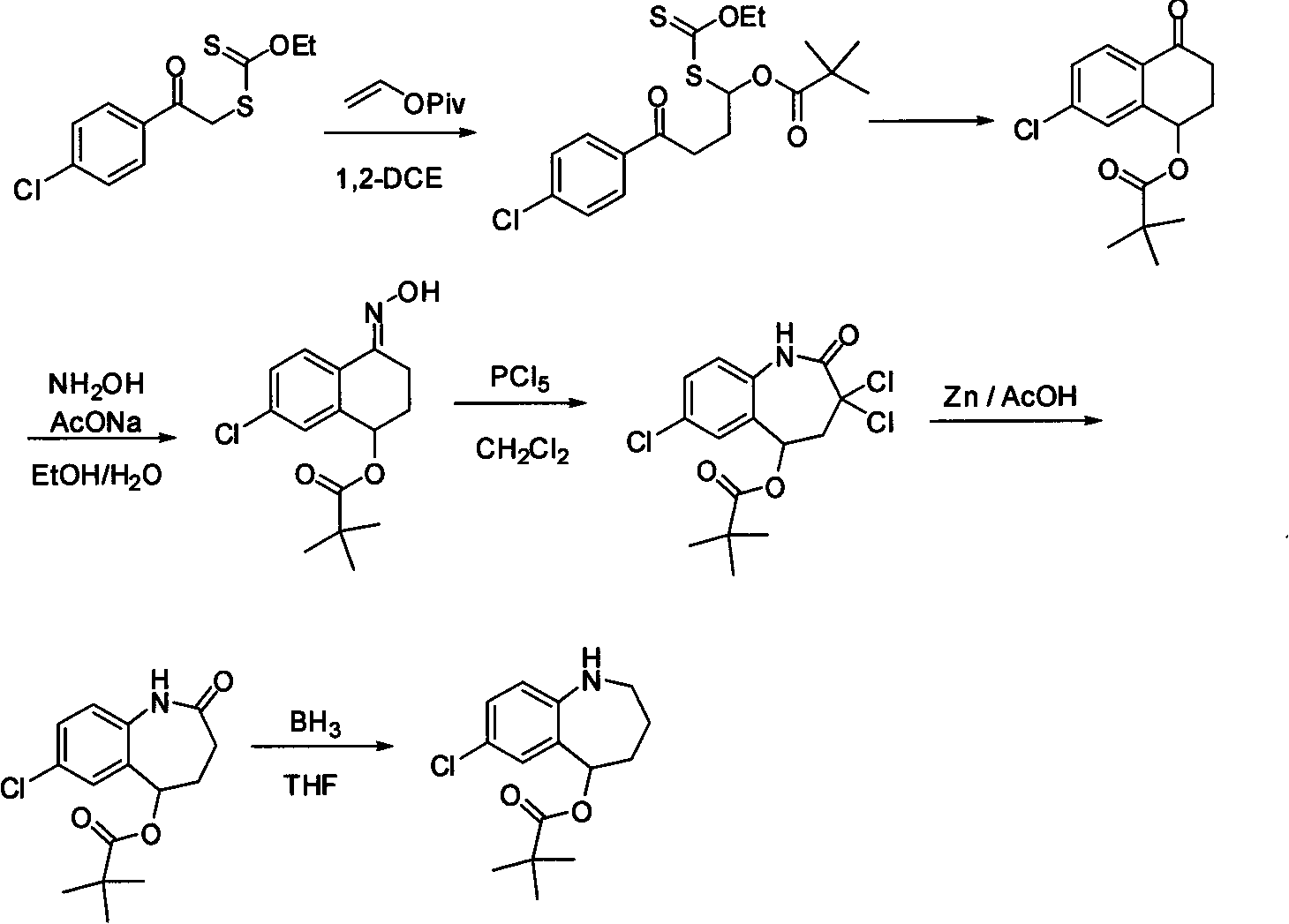
Method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone
The invention relates to a preparation method of benzazepine compounds, particularly a preparation method of 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone. The 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone can be used as an intermediate for preparing a pitressin antagonist medicament Tolvaptan. The method comprises the following steps: by using parachloroaniline as a raw material, reacting with paratoluensulfonyl chloride in a condensation mode under the alkaline condition to firstly protect amino groups; under the alkaline condition, coupling with ethyl 4-bromobutyrate to obtain a compound; in the presence of alkali, hydrolyzing the obtained compound, and acidifying; in dichloromethane, preparing acyl chloride from the acidified compound under the action of thionyl chloride; under the action of Lewis acid, carrying out Friedel-Crafts acylation reaction to perform intramolecular cyclization; and finally, removing tosyl groups to obtain the target product. The invention has the advantages of simple technique and low cost, and is convenient for industrial large-scale production.
Owner:宁波人健药业集团股份有限公司
Method for synthesizing mometasone furoate or monohydrate of mometasone furoate
ActiveCN106279340AThe generation of solutionNo generationSteroidsAfter treatmentMometasone furoate monohydrate
The invention belongs to a synthesizing method for medicine, and particularly relates to a method for synthesizing mometasone furoate or a monohydrate of the mometasone furoate. The method includes the steps that 8-DM serving as a first compound is used as an initial material, and the first compound and paratoluensulfonyl chloride are subjected to a sulfonylation reaction to generate a second compound; the second compound is not subjected to after-treatment and is subjected to a chlorination reaction with RCl (R is Li, Na, K and Et3N) to generate a third compound; the third compound is not subjected to after-treatment, a part of organic alkali is replenished, and the mixture and furoyl chloride are subjected to an esterification reaction to generate a fourth compound; the fourth compound is not subjected to after-treatment, acid adjustment is carried out, a large quantity of chlorine elements existing in a reaction system are used for a ring-opening reaction, and the mometasone furoate or the mometasone-furoate monohydrate is obtained. The method is simple in technology, mild in reaction condition, high in yield, low in cost, high in quality and raw-auxiliary-material using rate, free of genetic toxicity impurity generation and suitable for industrial production.
Owner:山东锐顺药业有限公司
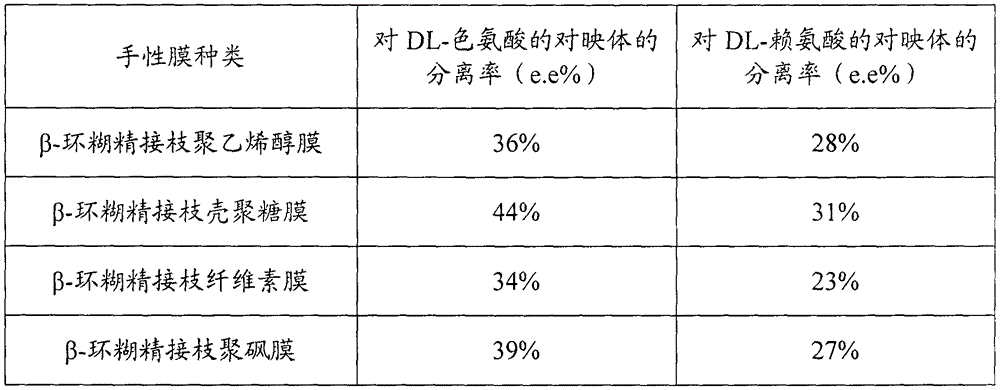
Beta-cyclodextrin grafted polymer chiral separation membrane and preparation method thereof
The invention belongs to the technical filed of polymer membrane separation and discloses a beta-cyclodextrin grafted polymer chiral separation membrane and a preparation method thereof. The method comprises the following steps: (1) with beta-cyclodextrin as the raw materials, preparing aldehyde cyclodextrin by reacting the beta-cyclodextrin with toluene sulfochloride and triethylamine in sequence; (2) performing aldolization with the aldehyde cyclodextrin and a polymer containing hydroxyl or Schiff base reaction with the aldehyde cyclodextrin and a polymer containing amidogen, so as to obtain a beta-cyclodextrin grafted polymer; and (3) dissolving the beta-cyclodextrin grafted polymer in a solvent to prepare a casting membrane liquid and preparing the beta-cyclodextrin grafted polymer chiral separation membrane by adopting a phase conversion method. The prepared beta-cyclodextrin grafted polymer chiral separation membrane can be applied for separating raceme, so as to realize green and efficient separation of chiral medicines.
Owner:TIANJIN POLYTECHNIC UNIV
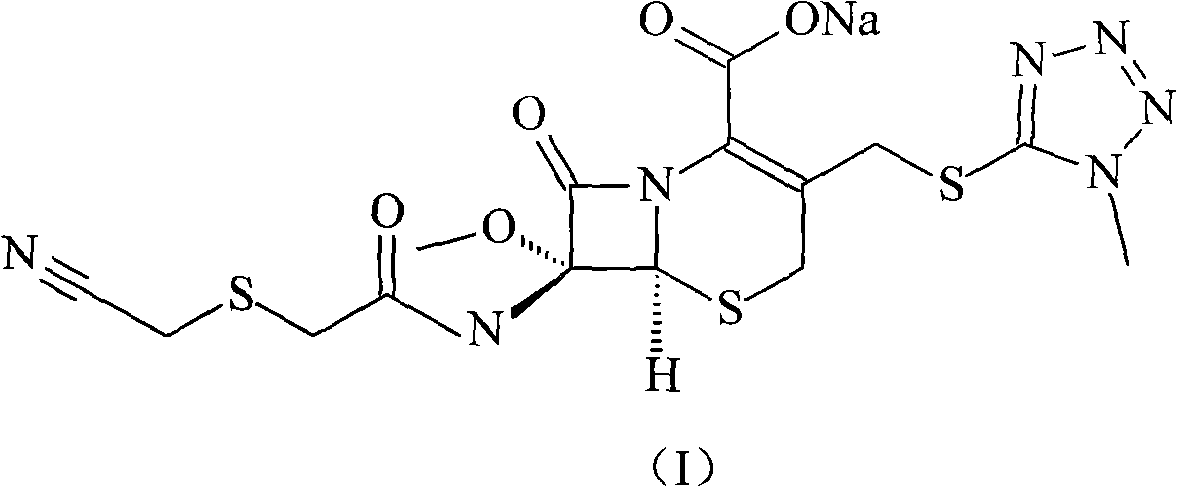
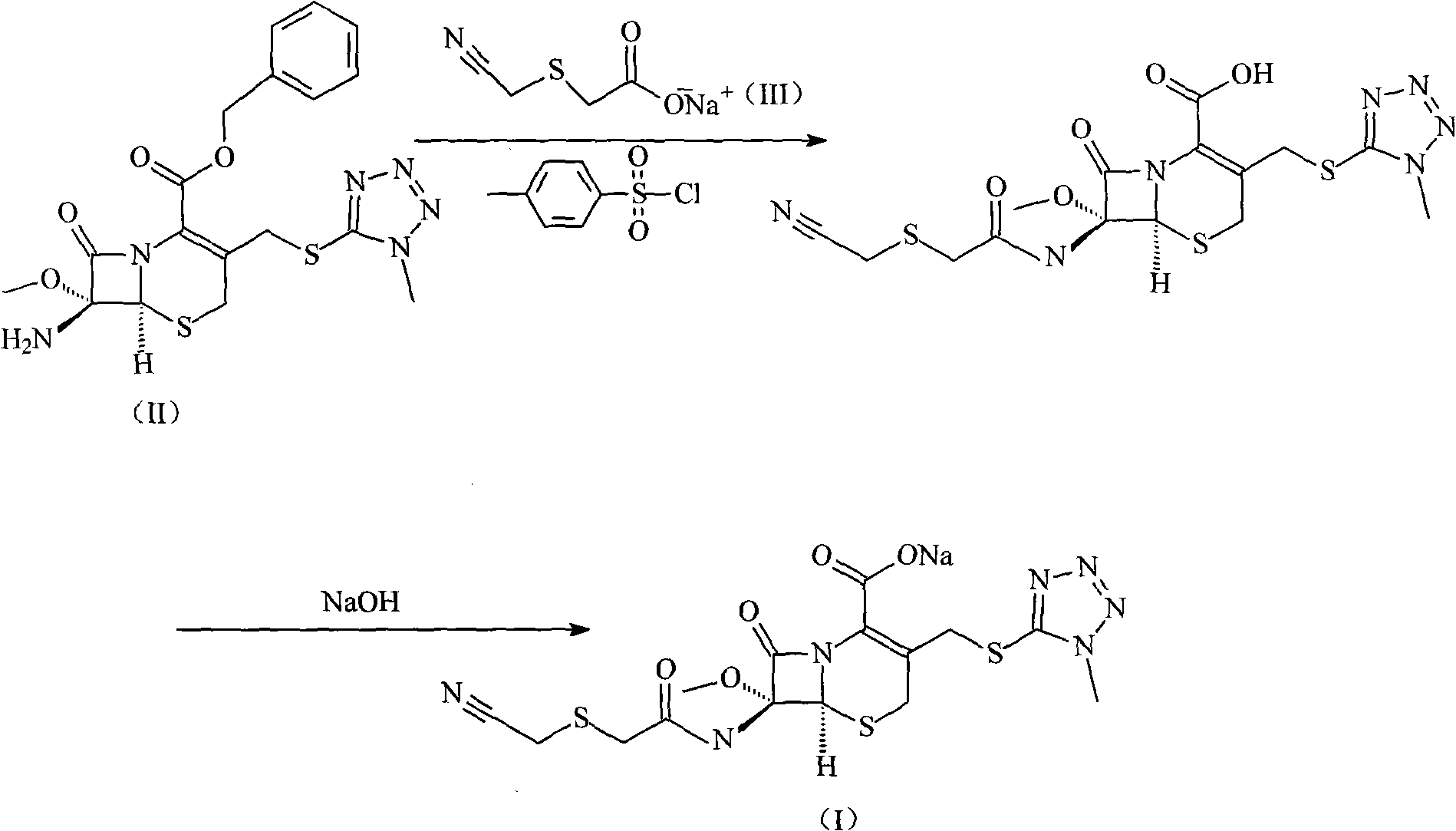
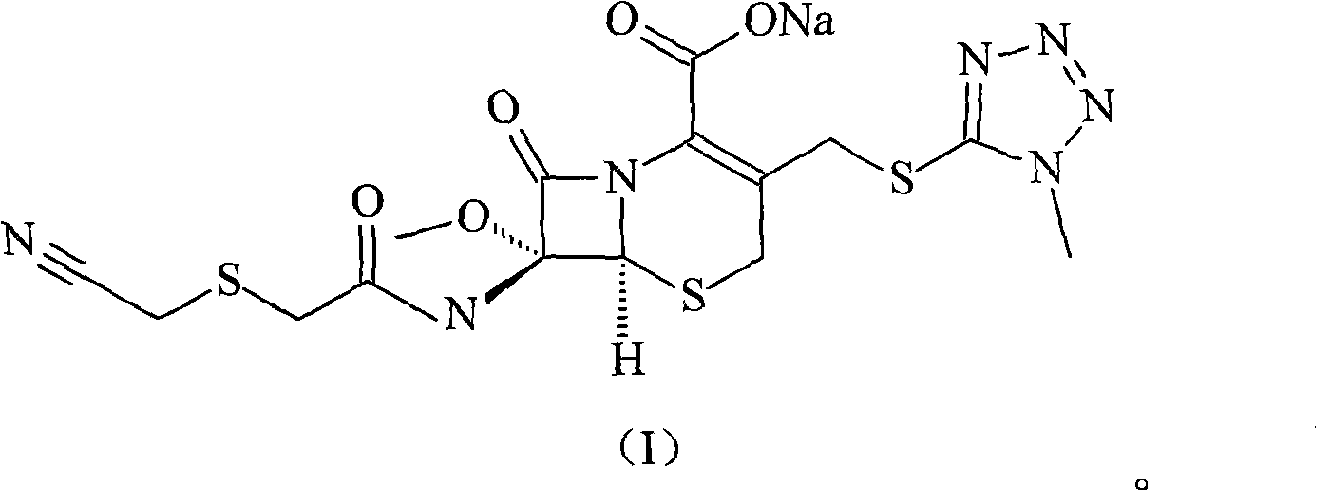
Cefmetazole sodium compound and synthetic method thereof
InactiveCN101550151AFew reaction stepsHigh yieldAntibacterial agentsOrganic chemistryAcetic acidCarboxylic salt
The invention relates to a cefmetazole sodium compound and a synthetic method thereof, 7 Beta-amino-7 Alpha-methoxyl-3-(1-methyl-1H-tetrazole-5-sulfidomethyl)-3-cephem-4-benzyl carboxylate and cyanomethylthio acetic acid sodium are mixed and react in the presence of p-toluenesulfonyl chloride to generate cefmetazole; sodium hydroxide is added to obtain cefmetazole sodium. Compared with the prior art, the invention has the advantages of few reaction steps, high productive rate, high product purity and low cost of raw materials, and has a broad prospect.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method of methyl p-tolyl sulfone
InactiveCN101659635AHighlight substantiveSignificant progressOrganic chemistryOrganic compound preparationSodium bicarbonateMethylating Agent
The invention relates to a preparation method of methyl p-tolyl sulfone, which belongs to the technical field of sulphone preparation, in particular to a preparation method of the methyl p-tolyl sulfone by taking monochloro methane as a methylating agent for synthesis. The preparation method is characterized by taking p-toluenesulfonylchloride, anhydrous sodium sulfite, sodium bicarbonate and monochloro methane as synthesizing raw materials and comprising the following operation steps: a. salifying the p-toluenesulfonylchloride; b. synthesizing the methyl p-tolyl sulfone; c. adjusting pH, reducing the temperature and discharging; d. filtering, dehydrating and drying; and e. inspecting the quality and packaging and warehousing after qualification. The invention provides a preparation methodof the methyl p-tolyl sulfone without using a deadly poisonous compound of dimethyl sulfate as a raw material, and has safe operation, environmental protection, short production period, good productquality, high yield, low production cost and strong market competitiveness. The content of the methyl p-tolyl sulfone reaches up to 99.5 percent, and the yield of the methyl p-tolyl sulfone, which iscounted by the p-toluenesulfonylchloride of the raw material, is more than or equal to 85 percent.
Owner:SHANDONG XINGHUI CHEM
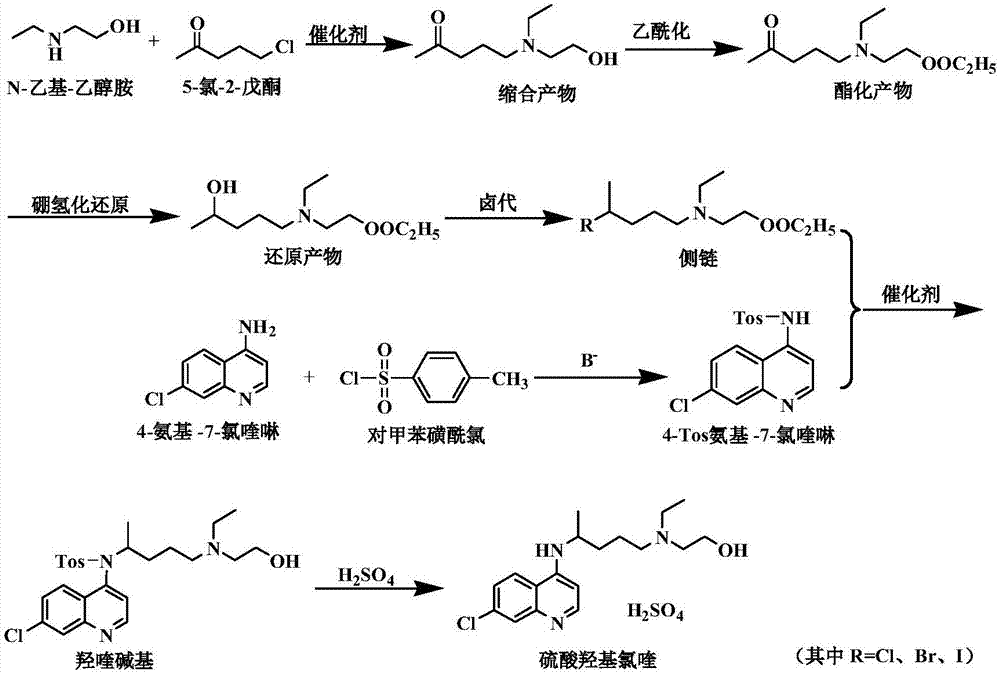
Side chain, synthesis method thereof, and method for synthesizing hydroxychloroquine sulfate from side chain
ActiveCN107266323AEasy to operateReduce the difficulty of industrial productionOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsSide chain
The invention discloses a side chain, a synthesis method thereof, and a method for synthesizing hydroxychloroquine sulfate from the side chain. The synthesis method of the side chain comprises the following steps: 1, condensing N-ethylethanolamine and 5-chloro-2-pentanone to obtain a condensation product; 2, esterifying the condensation product and an acetyl reagent to obtain an esterification product; 3, reducing the esterification product to obtain a reduction product; and 4, reacting the reduction product with a halogenating agent to obtain the side chain. The synthesis method of the hydroxychloroquine sulfate comprises the following steps: 1, reacting 4-amino-7-chloroquinoline with paratoluensulfonyl chloride to obtain 4-Tos-amino-7-chloroquinoline; 2, reacting the side chain with the 4-Tos-amino-7-chloroquinoline to obtain a hydroxyquine base; and 3, reacting the hydroxyquine base with sulfuric acid to obtain the hydroxychloroquine sulfate. The synthesis method of the new side chain avoids the ammonification process and the catalytic hydrogenation process, and is safe and environmentally friendly, and the hydroxychloroquine sulfate can be obtained through low-temperature condensation of the side chain, so the quality of the above products is remarkably improved, and the production flow is simplified.
Owner:宜宾莱特医药化工有限公司
Photosensitive chiral macrocyclic molecule and preparation method and application thereof
ActiveCN104496933AAchieve complexationAchieve releaseLiquid crystal compositionsOrganic chemistryN dimethylformamideCis trans isomerization
The invention discloses a photosensitive chiral macrocyclic molecule and a preparation method and application thereof, belonging to the field of preparation of photoresponsive materials. The preparation method for the photosensitive chiral macrocyclic molecule adopts the following steps: a) reacting oligomeric ethylene glycol, sodium hydroxide and p-toluenesulfonyl chloride in a tetrahydrofuran solvent in an inert atmosphere so as to obtain an intermediate 1; b) dissolving the intermediate 1, cesium carbonate and dibenzo-18-crown-6 in an N-N dimethylformamide solvent and carrying out a reaction in an inert atmosphere so as to obtain an intermediate 2; and c) dissolving the intermediate 2, chiral binaphthol compounds, cesium carbonate and dibenzo-18-crown-6 in the N-N dimethylformamide solvent and carrying out a reaction in an inert atmosphere so as to obtain a target product. The photosensitive chiral macrocyclic molecule provided by the invention has the advantages of easy synthesis, good stability, ability of selective complexation of chiral ammonium salt and capability of realizing complexation and release of chiral ammonium salt through an azo cis-trans isomerization behavior.
Owner:黄山市开发投资集团有限公司
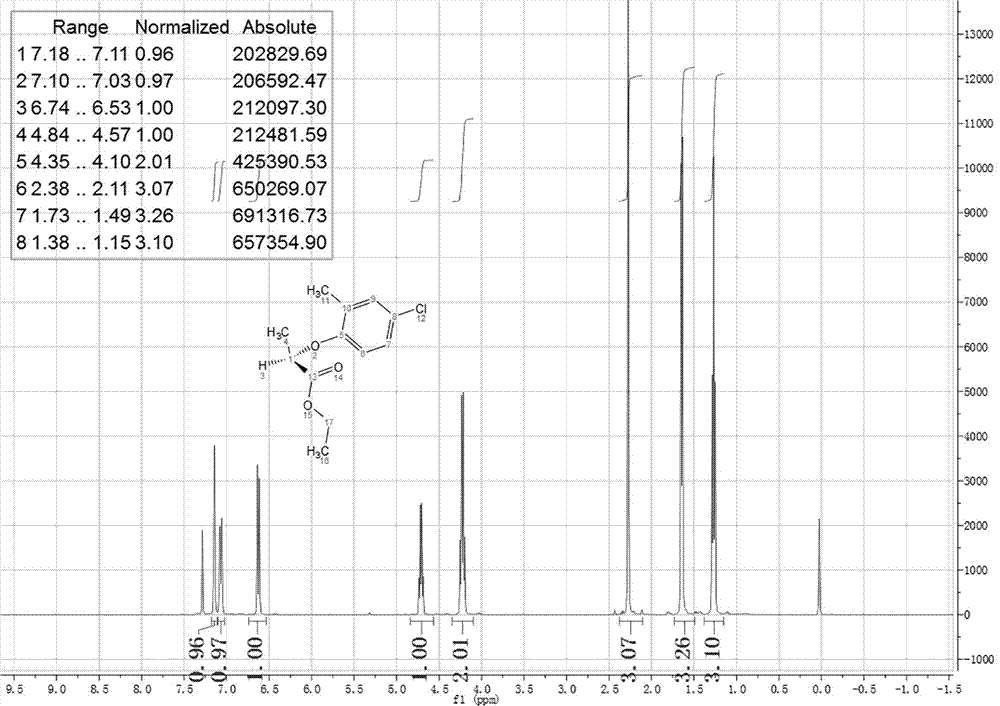
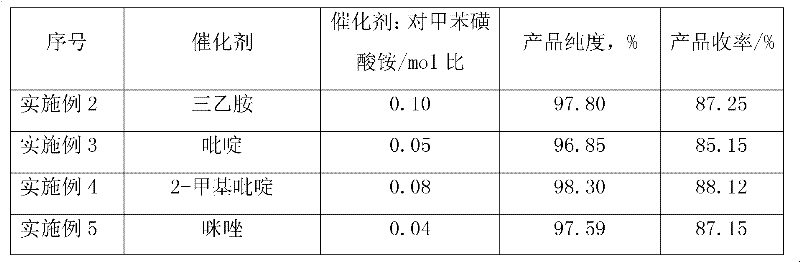
Method for preparing paratoluensulfonyl chloride
The invention discloses a method for preparing paratoluensulfonyl chloride, which comprises the following step of: reacting paratoluenesulfonic acid ammonium salt with bis(trichloromethyl) carbonate (commonly called triphosgene) in an inert organic solvent under the condition that organic alkali is used as a catalyst to synthesize the paratoluensulfonyl chloride. The preparation method has the advantages that: raw materials are conveniently and readily available, the process is simple and suitable for scale-up production, reaction conditions are mild, and a product is easy to purify. The prepared paratoluensulfonyl chloride is an important fine chemical product and can be used for preparing a dye intermediate, synthesizing intermediates of more than ten kinds of antibacterial medicines and anti-inflammatory medicines such as betamethasone, sulfamylon and the like and synthesizing plastic plasticizers, resin, coatings, pesticides and light-sensitive materials.
Owner:SINOCHEM LANTIAN +1
Method for synthesizing 24-epibrassinolide
ActiveCN111004303ASuitable for industrial productionReduce pollutionSteroidsSulfonyl chlorideOrganic synthesis
The invention belongs to the technical field of organic synthesis. Based on a seven-step synthesis process, p-toluene sulfonyl chloride is used as an acylation reagent; a manganese dioxide / air catalytic system is used in a water and methanol system; and a calcium / HMPA / R1(CH2)nR2 reduction system is used. According to the synthesis method of the 24-epibrassinolide, ergosterol is used as a startingraw material, a seven-step synthesis process is adopted, the method comprises esterification, hydrolysis, oxidation, reduction, rearrangement, dihydroxylation and lactonization; methylbenzene is usedas a solvent in the esterification process, and ergosterol reacts with benzene sulfonyl chloride in the presence of an acid-binding agent; wherein a manganese dioxide / air system is adopted in the oxidation process, methanol is used as a solvent, and oxidation is performed in air; and a calcium / R1(CH2)nR2 / / HMPA / reduction system is adopted in the reduction process. According to the synthesis method,the total yield is increased by 20 points or above, the product quantitative content is 85% or above, the reaction condition is mild, the technological process is short, the operation is convenient,manganese dioxide can be recycled, low toxicity and low environmental pollution are achieved, and the synthesis method is suitable for industrial production of 24-epibrassinolide.
Owner:JINGBO AGROCHEM TECH CO LTD
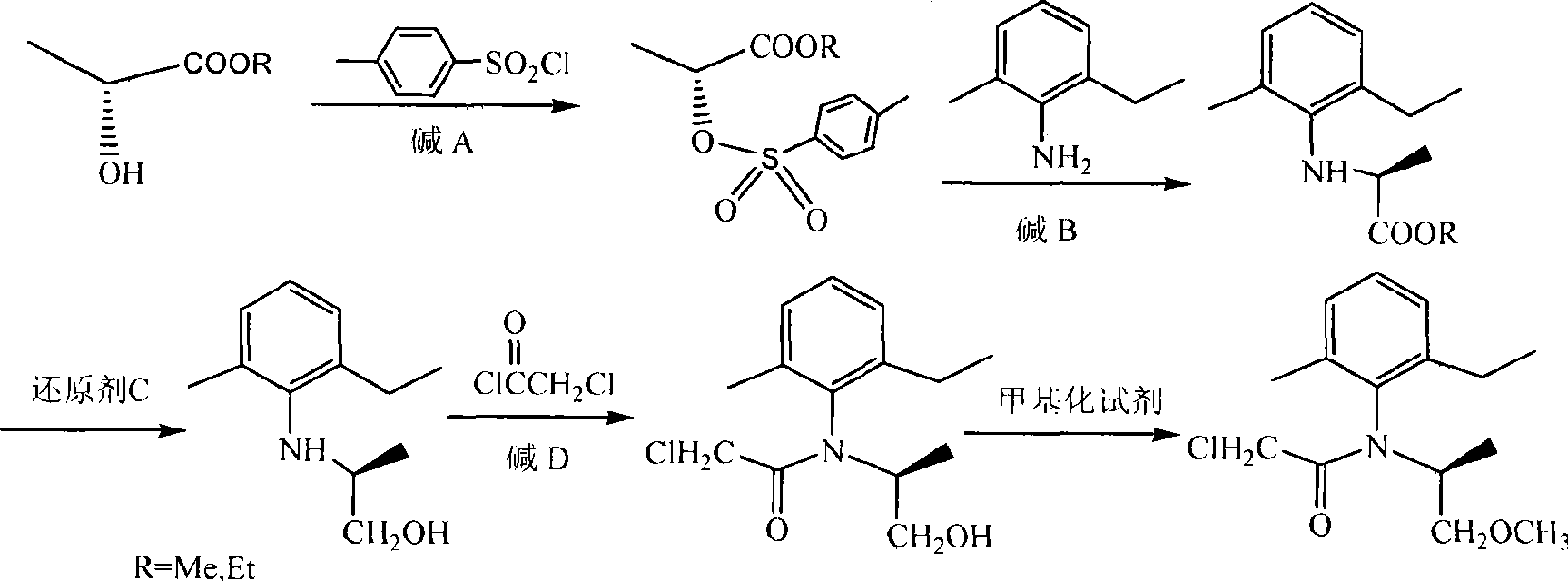
Novel method for synthesis of (S)-propisochlor
ActiveCN101367746ALow costMild responseOrganic compound preparationCarboxylic acid amides preparationMethyl lactateAniline
The present invention relates to a novel method of synthesizing (S)-metolachlor, and discloses a complete synthesis route which adopts chiron. In the novel method, (D)-methyl lactate or (D)-ethyl lactate is used as a raw material and reacts with p-toluenesulfonyl chloride to generate (R)-2-(p-tolylsulfonyl oxyl) propionate; the (R)-2-(p-tolylsulfonyl oxyl) propionate reacts with 2-methyl-6-ethyl aniline to prepare S-(-)-N-(2-methyl-6-ethyl benzyl) alanine ester; the S-(-)-N-(2-methyl-6-ethyl benzyl) alanine ester reacts with a reducing agent C to prepare S-(-)-N- (1'-methyl-2'-ethylol)-2-methyl-6-ethyl aniline; and the S-(-)-N- (1'-methyl-2'-ethylol)-2-methyl-6-ethyl aniline is acylated with chloroacetic chloride and ultimately methylated to prepare the (S)-metolachlor. The present invention has the advantages that no resolution is required in the whole process, the reaction is mild, the yield rate is high, the optical purity is good, the reaction time is short, the raw materials are inexpensive and can be easily acquired, and the cost is low.
Owner:NANJING UNIV OF TECH +1
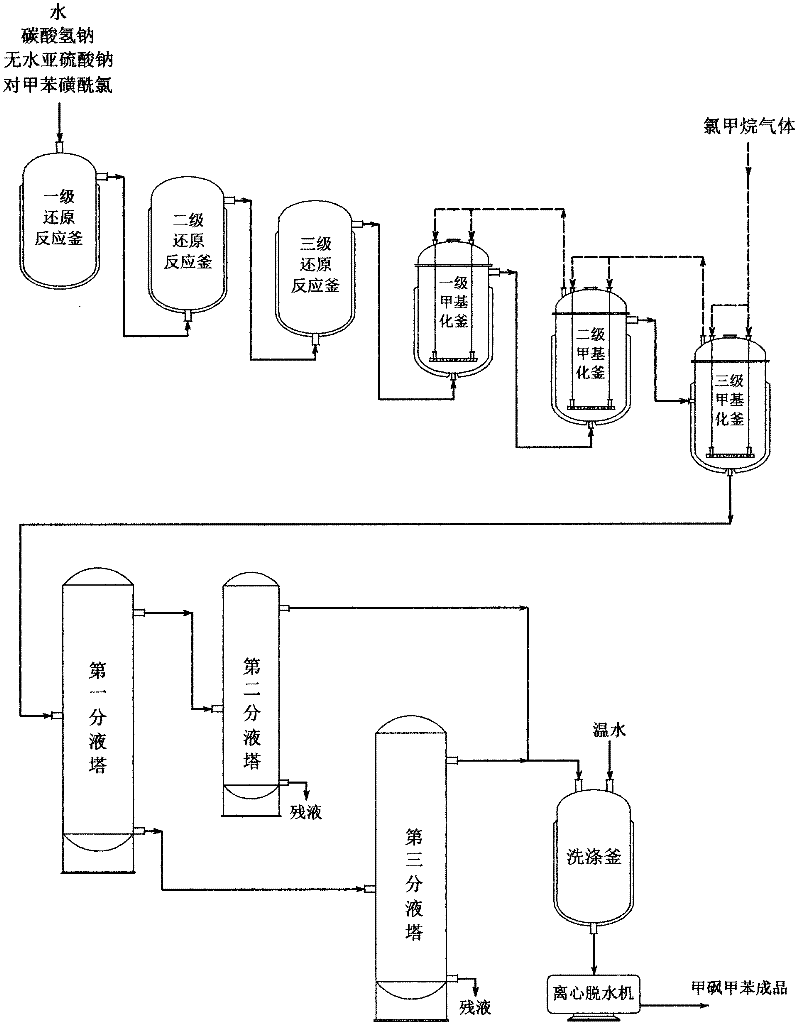
Industrialized production method for methyl p-tolyl sulfone
ActiveCN102363604AAvoid harmRealize continuous productionOrganic chemistryOrganic compound preparationSodium bicarbonateOrganic layer
An industrialized production method for methyl p-tolyl sulfone comprises the following steps of: carrying out reduction reaction to p-toluenesulfonyl chloride and anhydrous sodium sulphite as well as sodium bicarbonate in a tertiary serial reduction reaction kettle, generating p-methyl sodium sulphinate; methylating the p-methyl sodium sulphinate into the methyl p-tolyl sulfone by chloromethane in a tertiary serial methylation kettle; separating an organic layer by a tertiary liquid separating tower; and preparing a methyl p-tolyl sulfone product through washing, crystallizing and dewatering. In the invention, dimethyl sulfate as a highly toxic chemical is not adopted to avoid the harm to human bodies and environment; and as a multi-kettle serial technology is adopted for reduction salification reaction and methylation reaction, p-toluenesulfonyl chloride salification and methyl p-tolyl sulfone synthesis can be completely reacted to realize continuous production.
Owner:浙江嘉化新材料有限公司
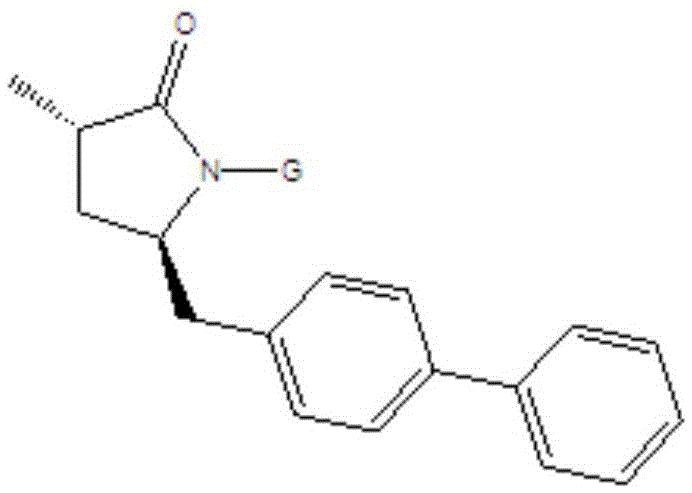
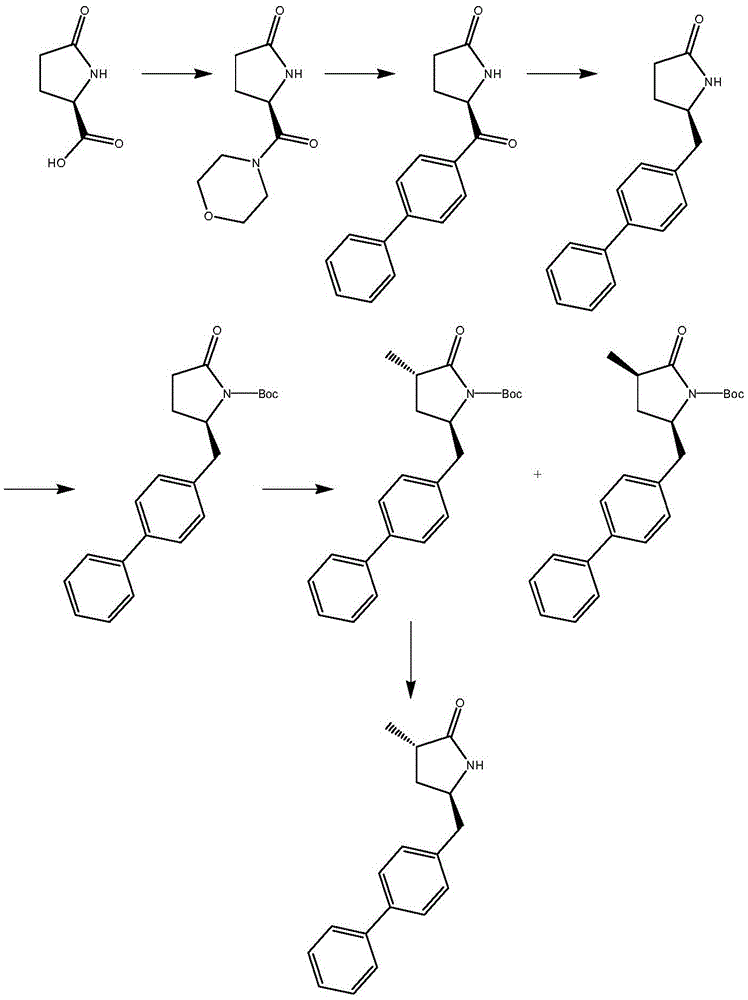
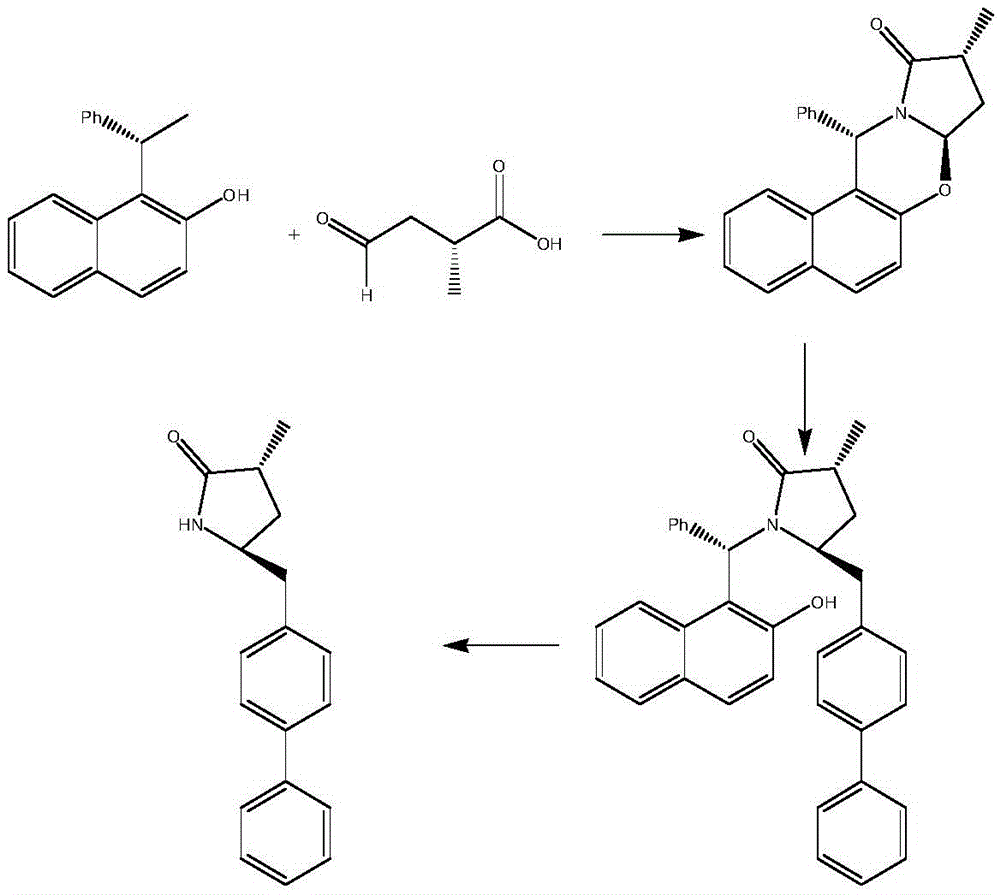
Preparation method for Sacubitril intermediate
InactiveCN105566194AHigh purityHigh yieldOrganic chemistry methodsPhenylmagnesium bromideMethyl methanesulfonate
The invention relates to a preparation method for a Sacubitril intermediate. The preparation method comprises the following steps that (3R,5S)-5-(hydroxymethyl)-3-methyl-2-pyrrolidone is esterified with toluene sulfochloride or methanesulfonyl chloride to obtain (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone; (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone is coupled with 4-diphenylmagnesium bromide or 4-diphenylmagnesium chloride to obtain (3R,5S)-3-methyl-5-(1,1'-diphenyl-4-yl-methyl)-2-pyrrolidinone. According to the preparation method, the method is novel, the raw materials are easy to obtain, the technology is simple, and the purity and yield of the product are both very high.
Owner:张伯引
Preparation method of tiamulin base
InactiveCN102675172AHarm reductionReduce salt formation and crystallization stepsSulfide preparationReaction temperatureKetone
The invention relates to a preparation method of tiamulin base. The preparation method includes: filtering pleuromulin fermentation broth, drying mycelium, performing leaching by using methanol as solvent, using acetone to extract leach liquor after vacuum concentration, concentrating extract, transferring concentrate into a synthetic reactor, adding paratoluensulfonyl chloride, allowing for synthetic reaction at pH of 11-12 and reaction temperature of 20-30 DEG C, allowing for standing and layering after complete reaction, adding tetrabutylammonium bromide and diethylaminoethyl mercaptide into ketone phase respectively , allowing for full reaction at pH of 9-10 and the reaction temperature of 50-60 DEG C, adding water for extraction, discarding aqueous phase, filtering the ketone phase, and reducing the original volume to 60-70% by vacuum concentration to obtain tiamulin base. The original process is improved, the salifying and crystallization process is omitted, the tiamulin base is used to produce preparations directly, and accordingly equipment investment cost and production cost are lowered, product quality is improved, and the content of tiamulin base reaches 80-90%. Organic solvents used in the preparation method are recyclable, environmental pollution is reduced, and the preparation method has promising development prospect.
Owner:宁夏泰瑞制药股份有限公司
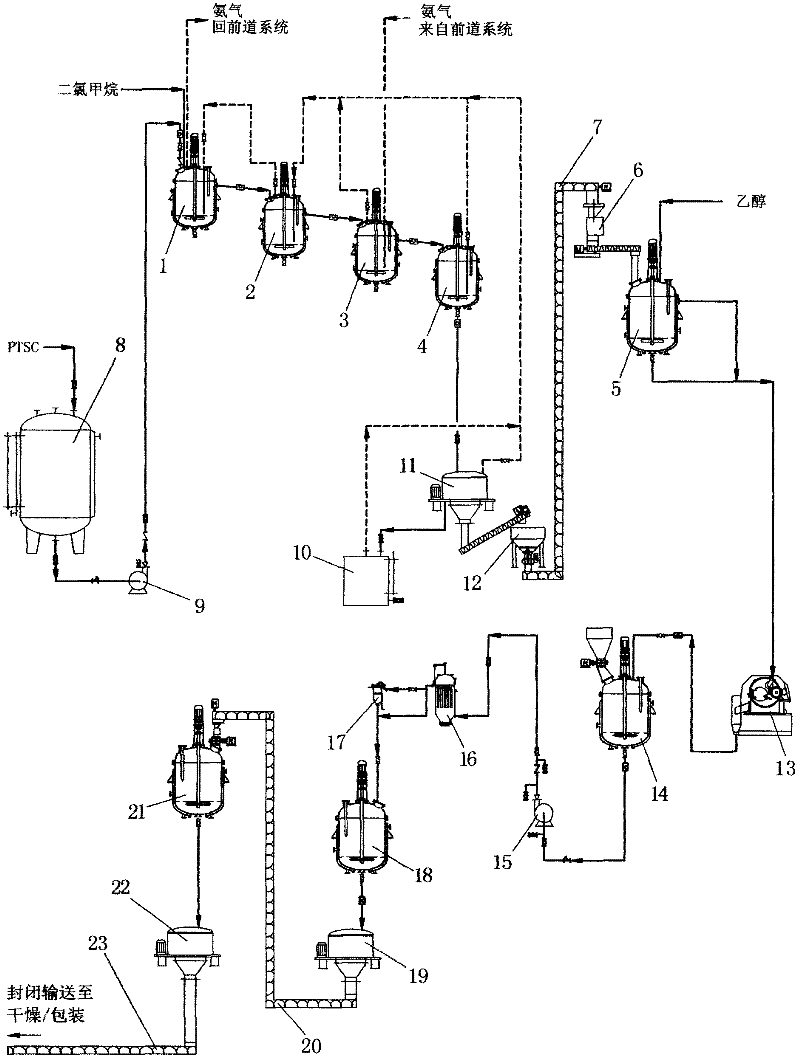
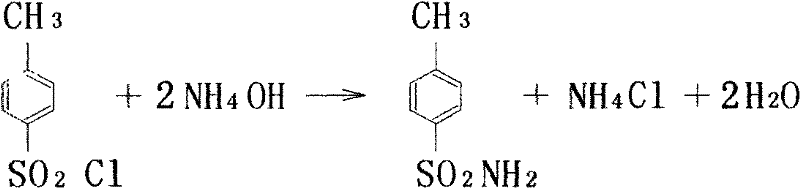
Industrial production method for toluene sulfonamide
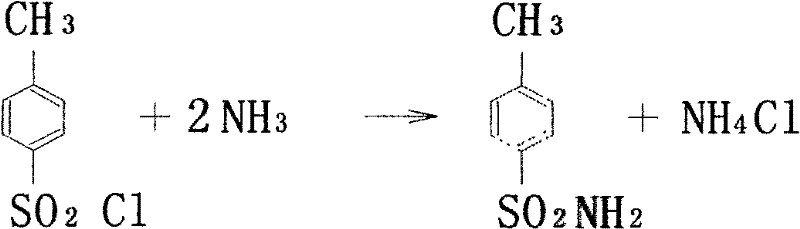
ActiveCN102584647AReduce pollutionReduce labor intensityOrganic compound preparationSulfonic acid amide preparationMethylene DichlorideSide reaction
The invention discloses an industrial production method for toluene sulfonamide. Continuous amination is performed on paratoluensulfonyl chloride and ammonia gas in methylene dichloride solvent in a countercurrent absorbing way, then active carbon is used for decoloring, and through alcohol recrystallization and drying after water scrubbing, a qualified product can be obtained. Meanwhile, continuous production is achieved, that gas ammonia in tail gas is discharged within controlling index of national standards can be achieved, environment pollutions are reduced, and meanwhile, labor intensity is lowered. Solvent is adopted for replacing water for amination, the side reaction of hydrolyzation is stopped, and COD emission in waste water is lowered greatly while reaction conversion rate is improved.
Owner:浙江嘉化新材料有限公司
Synthesis process of tolylsulfonyl chloride
The present invention is the new synthesis process of tolylsulfonyl chloride with toluene as material, chlorosulfonic acid and phosphorus oxychlorides as sulfonating agent and inorganic ammonium slat as sulfonating assistant. Under the action of sulfonating agent chlorosulfonic acid and phosphorus oxychloride and sulfonating assistant, the material toluene is sulfochlorinated to produce the reacted liquid; and the reacted liquid is made to pass through low temperature hydrolysis, solvent extraction, water washing, eliminating water layer, crystallization of the oil phase at temperature lower than 5 deg.c, filtering and drying to obtain solid tolylsulfonyl chloride. The filtrate is decompression distilled to eliminate solvent to obtain sulfonated oil with o-tolylsulfonyl chloride as main component with total yield as high as 98.8%.
Owner:ZHEJIANG UNIV
Chemical synthesis method of valnemulin hydrochloride
The invention discloses a chemical synthesis method of valnemulin hydrochloride, which comprises the following steps of: taking refined pleuromutilin as raw material, carrying out sulfonation by paratoluensulfonyl chloride, and reacting with dimethyl cysteamine hcl, to obtain the pleuromutilin dimethyl cysteamine substitute; reacting D-valine, methyl acetoacetate and potassium hydroxide to obtain (R)-2-(1-methoxycarbonyl group-2-allyl) amino-3-methyl potassium butyrate, activating by ethyl chloroformate and reacting with the pleuromutilin dimethyl cysteamine substitute, adjusting PH value, carrying out reverse phase extraction, and carrying out freeze-drying to obtain the valnemulin hydrochloride. The method has the advantages that due to the refining of the raw material pleuromutilin, the impurities in the product can be effectively removed, and the purifying process can be simplified from the source; the carboxyl of D-valine can be activated by the ethyl chloroformate, so that the reaction is easier to carry out; and due to the pH adjustment, the reverse phase extraction, and the freeze-drying, the product can be obtained, so that the product is stable in quality, and high in purity.
Owner:武汉回盛生物科技股份有限公司
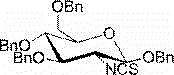
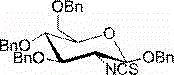
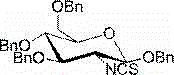
2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose, and synthetic method and application thereof
ActiveCN104961781AEasy to separate and purifySimple and fast operationSugar derivativesSugar derivatives preparationSulfonyl chloridePyranose
The invention relates to 2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose. The invention also relates to a synthetic method for 2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose. The synthetic method comprises the following concrete steps: reacting 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl-beta-D-pyranose hydrochloride and carbon disulfide with triethylamine; and then reacting a reaction product with p-toluene sulfonyl chloride so as to obtain 2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose, wherein acetonitrile is used as a solvent for the reaction, reaction temperature is 0 DEG C, and reaction time is 1 to 2 h. The synthetic method has the advantages of easy and safe operation, small environmental pollution, high yield and simple post-treatment. The prepared 2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose can be used as an organic synthesis intermediate for synthesis of a glucosamine derivative.
Owner:HUAIHAI INST OF TECH
5,10-dihydroindolo[3,2-b]indole derivative synthesis method
InactiveCN105859725AEasy to operateReaction raw materials are readily availableOrganic chemistrySynthesis methodsNitrogen gas
The invention discloses a 5,10-dihydroindolo[3,2-b]indole derivative synthesis method. The method includes: (1) in a triethylamine solution of 2-iodoaniline derivatives (IV) and 2-acetenyl aniline derivatives (V), adding copper iodide and bis(triphenylphosphine)palladium chloride under nitrogen protection, and heating and refluxing under nitrogen protection to generate 2,2'-(acetenyl-1,2-2-yl) diphenylamine derivatives (III); (2) reacting the compounds (III), pyridines and paratoluensulfonyl chloride to generate compounds (II) at the room temperature; (3) reacting the compounds (II) and copper acetate in dimethylformamide to obtain 5,10-dihydroindolo[3,2-b]indole derivatives (I). The 5,10-dihydroindolo[3,2-b]indole derivative synthesis method has advantages of simplicity in operation, easiness in acquisition of reaction raw materials, mild reaction conditions, realization of substituent type diversity, wide substrate application range and the like.
Owner:TIANJIN UNIV
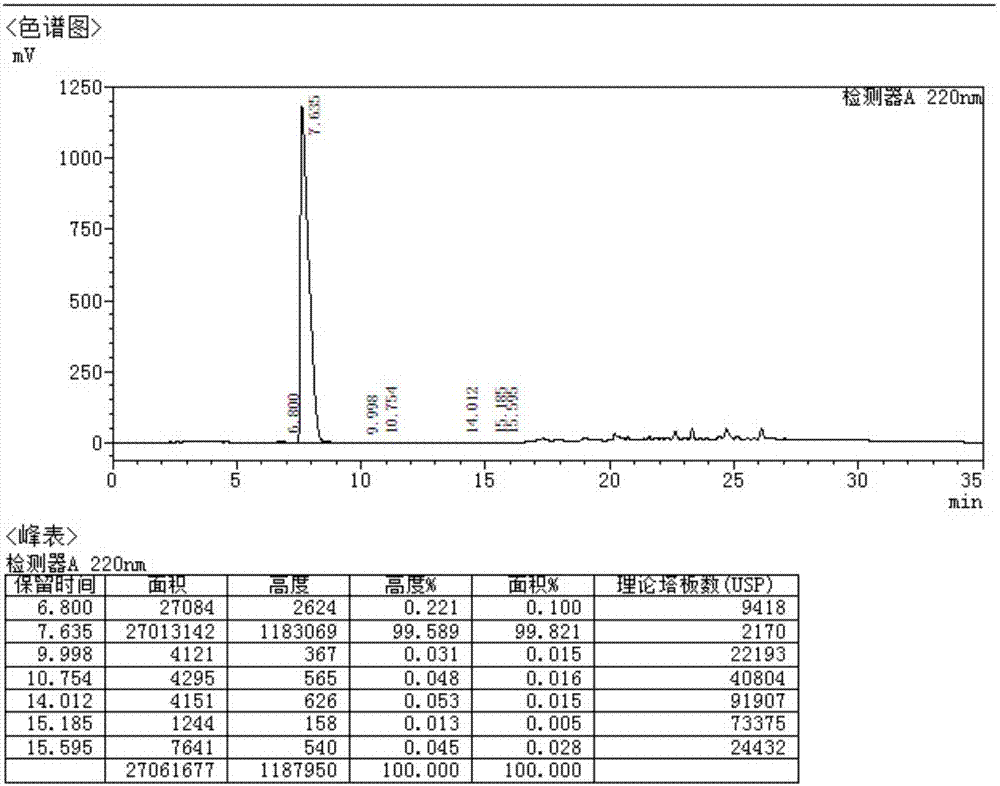
Method for measuring optical purity of (R)-3-quinuclidinol
ActiveCN104133018AEasy to identifyHigh purityComponent separationBenzenesulfinic acidMethyl palmoxirate
The invention belongs to the technical field of pharmaceutical chemical engineering, and in particular relates to a method for measuring the optical purity of (R)-3-quinuclidinol. The method for measuring the optical purity of (R)-3-quinuclidinol comprises the steps of (a) preparing a sample; (b) preparing a test solution; (c) preparing a chromatographic condition, and performing detection. According to the method, a precolumn derivatization chiral chromatography method is adopted to measure the optical purity of (R)-3-quinuclidinol; specifically, the precolumn derivatization method is used for enabling (RS)-3-quinuclidinol racemate and paratoluensulfonyl chloride to react to generate (RS)-3-quinuclidinol paratoluensulfonyl chloride derivative, namely quinuclidinol 3-paratoluene sulfonate; then the chiral chromatography method is used for measuring the optical purity; therefore, the technical difficulty in measurement of the optical purity of (R)-3-quinuclidinol is creatively solved.
Owner:JINAN ASIA PHARMA TECH
Method for synthesis of p-carboxybenzene sulfonamide through catalytic oxidation
ActiveCN104447434ANo pollution in the processUser-friendlySulfonic acid amide preparationHeteropoly acidCatalytic oxidation
The invention relates to a method for synthesis of p-carboxybenzene sulfonamide through catalytic oxidation, belonging to a method for preparing p-carboxybenzene sulfonamide. According to the method, p-carboxybenzene sulfonamide is prepared by two-step reaction by taking p-toluenesulfonyl chloride as raw material. The method comprises the following steps: firstly performing ammonolysis on p-toluenesulfonyl chloride to obtain p-toluenesulfonamide, oxidizing p-toluenesulfonamide to obtain a product, namely p-carboxybenzene sulfonamide by taking a hydroperoxide as an oxidant to replace traditional strong oxidants, namely sodium bichromate, potassium permanganate and the like under the catalytic action of a metal oxide and heteropoly acid in mild conditions in a water phase, wherein the yield is 81.83-87.88%, the purity of the product detected by analysis of an Agilent 1200 type high performance liquid chromatographic instrument is 92.54-95.47% and the selectivity of p-carboxybenzene sulfonamide can achieve 100%. According to the method, the catalysts and the oxidant are non-toxic and environment-friendly, a byproduct is water or alcohol which is environment-friendly, the reaction conditions are mild, the post-treatment is simple, the catalysts can be recycled, and the cost is saved, so that the method is an ideal green synthesis method of p-carboxybenzene sulfonamide.
Owner:CHINA UNIV OF MINING & TECH
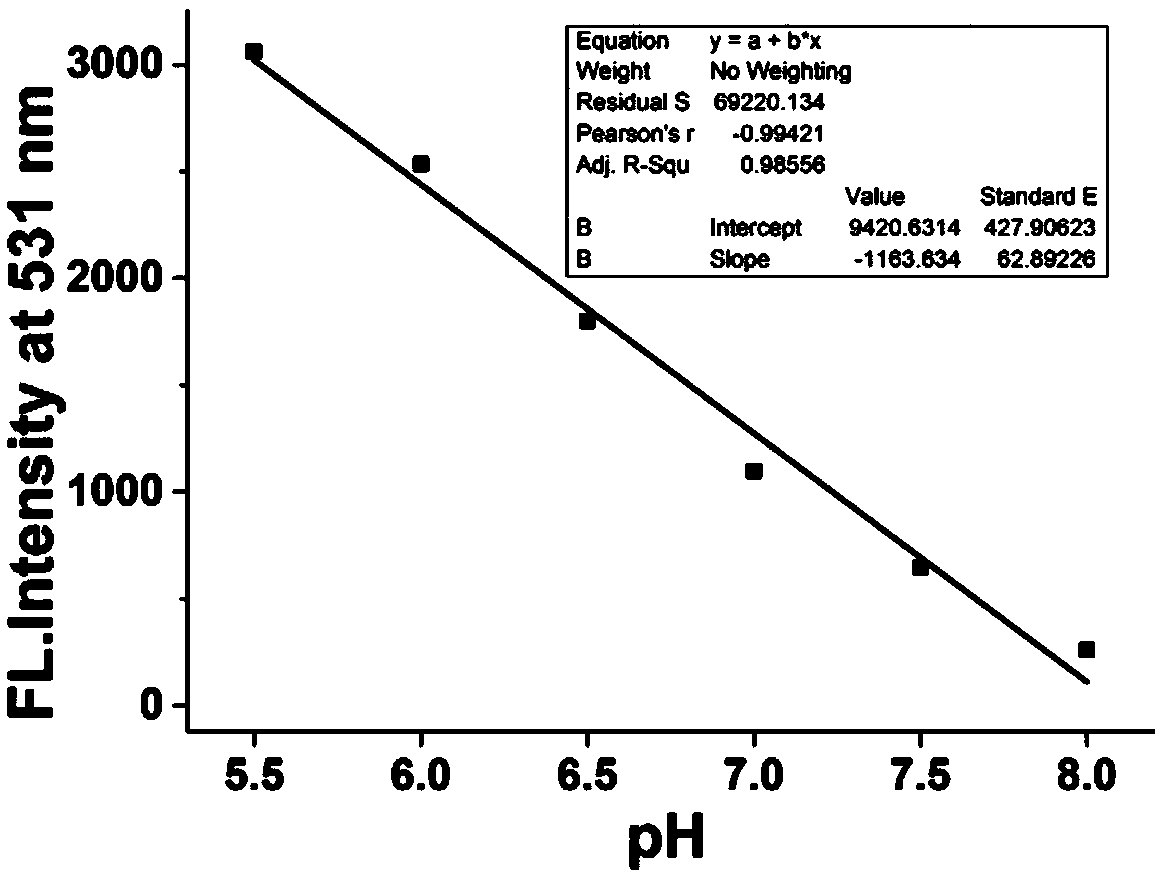
Two-photon fluorescent probe for detecting pH in endoplasmic reticulum of cell
InactiveCN109096189AStrong specificityHigh sensitivityOrganic chemistryFluorescence/phosphorescenceChemical synthesisFluorescence
The invention provides a fluorescent probe for detecting pH in endoplasmic reticulum of a cell. The formula is as shown in the description. The probe is prepared by carrying out three-step reactions by taking 4-bromo-1,8-naphthalic anhydride, BOC-ethanediamine, trifluoroacetic acid and paratoluensulfonyl chloride. The fluorescent probe can be used for detecting pH in a solution or the endoplasmicreticulum of the cell. The fluorescent probe can be obtained through chemical synthesis. The synthetic process is simple and feasible, the raw materials are cheap, the preparation cost is low, and theprobe is easy to popularize, high in specificity, is not affected by other components, has a two-photon property, can be used for detecting the pH in the endoplasmic reticulum of a living cell in a tissue and has a wide application prospect.
Owner:UNIV OF JINAN
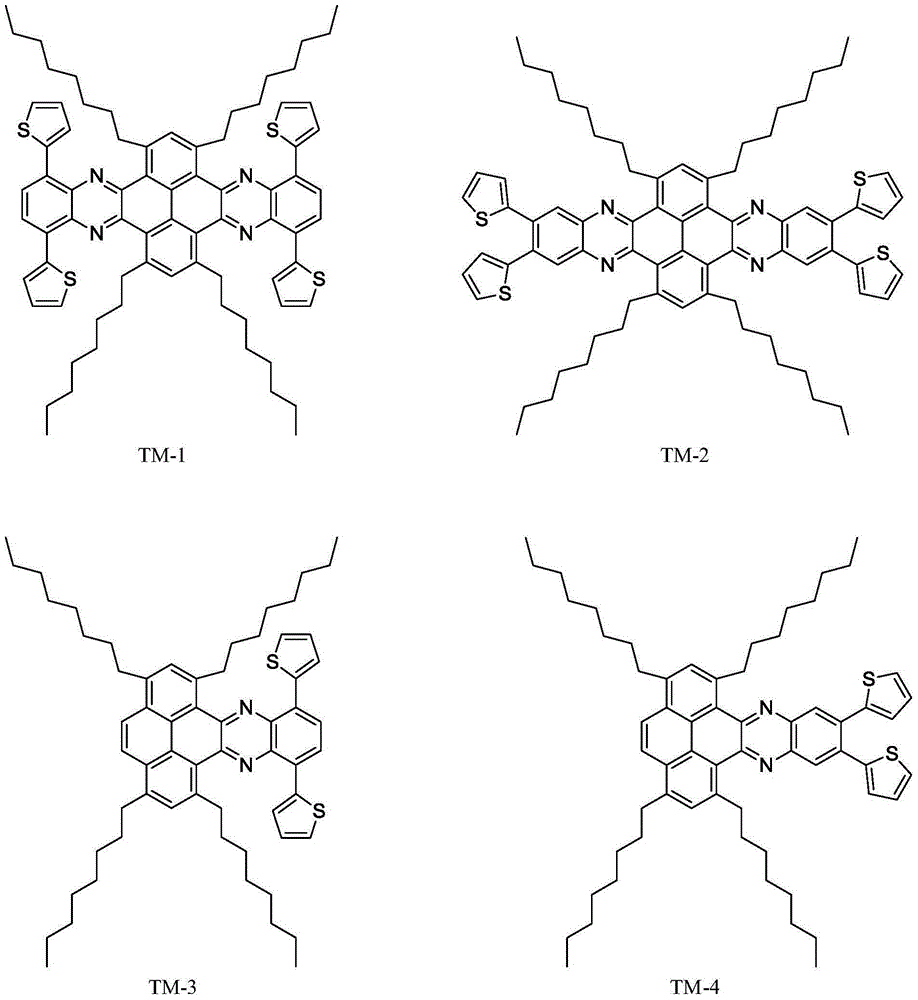
Thiophene polycyclic organic semiconductor material synthesis based on pyrene
InactiveCN105111216AImprove stabilityPlanar structureOrganic chemistrySemiconductor materialsSynthesis methods
The invention discloses thiophene polycyclic organic semiconductor material synthesis based on pyrene and belongs to the field of photoelectric conversion materials. The molecular formulas of compounds are C<76>H<86>N<4>S<4> and C<62>H<80>N<2>S<2>, and the structural formula can be seen in the Application of the invention. A synthesis method comprises the steps of making the pyrene react with liquid bromine, and obtaining 1,3,6,8-tetrabromo pyrene; making the 1,3,6,8-tetrabromo pyrene react with 1-octyne, obtaining a product, and then using palladium / carbon for conducting catalytic reaction,and obtaining 1,3,6,8-quadri-octane pyrene; using ruthenium trichloride for catalyzing the 1,3,6,8-quadri-octane pyrene, adjusting the using amount of sodium periodate, and obtaining tetrone and diketone; then making o-phenylenediamine and sulfoxide chloride react with liquid bromine, and obtaining 4,7-dibromo-2,1,3-diazosulfide; making thiophene react with thiadiazole, and obtaining diazosulfide; then making o-phenylenediamine and paratoluensulfonyl chloride react with liquid bromine, subsequently, making the mixture react with concentrated sulfuric acid, then making the mixture react with thionyl chloride, and obtaining 5,6-dibromo-2,1,3-diazosulfide; conducting operation same with the reaction process, and obtaining another type of diazosulfide; making a thiadiazole product to be subjected to ring-opening reaction, and obtaining two types of o-phenylenediamine products; making the tetrone and the diketone react with the two types of o-phenylenediamine respectively, and obtaining the compounds.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Preparation method of 2-(4'-methyl benzene sulfonyl) ethyl propionate
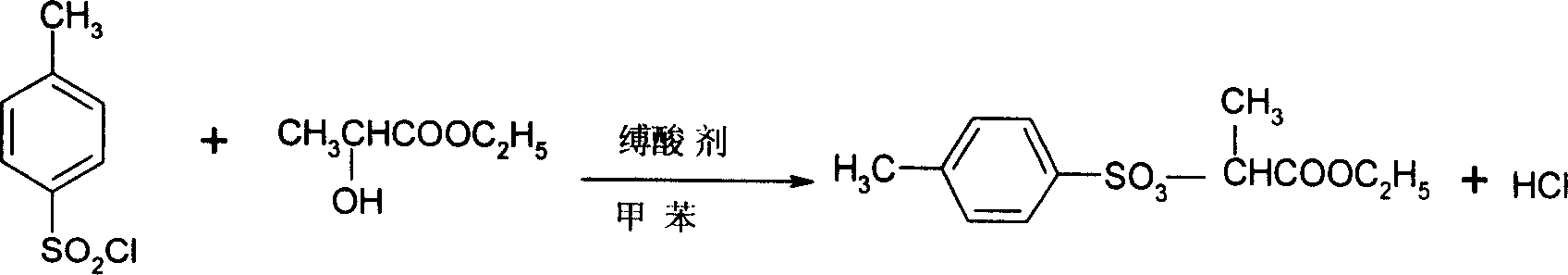
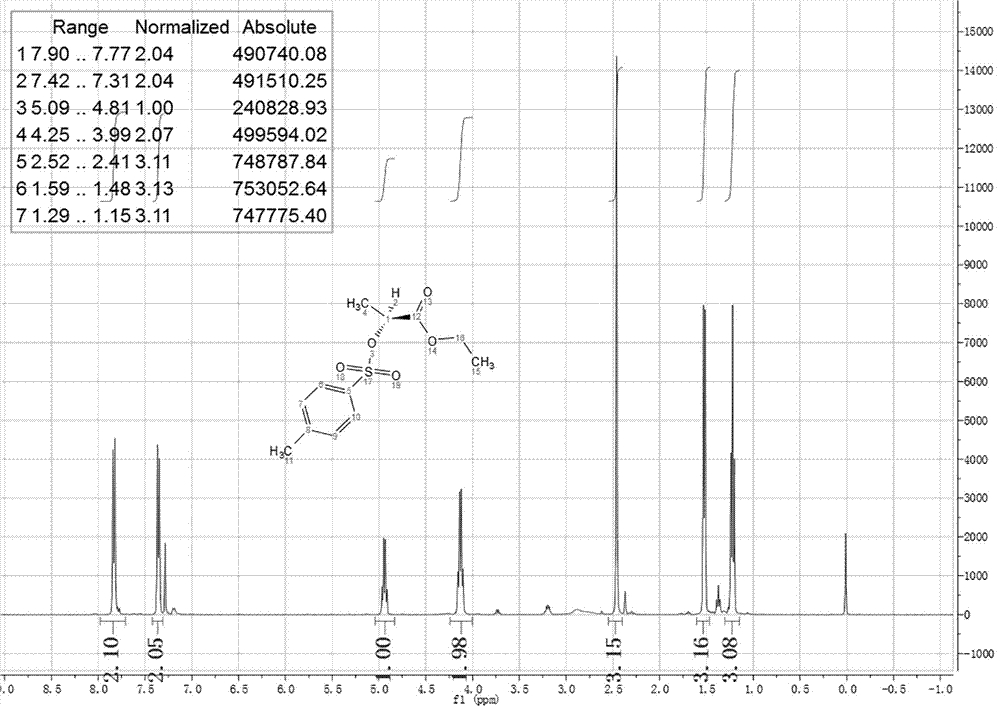
A process for preparing 2-(4'methylbenzosulfonyl) ethyl propionate includes such steps as adding toluene, P-toluene sulfuryl chloride and sodium hydroxide or potassium carbonate or CaO into reactor, dripping ethyl L-lactate at a temp lower than 10 deg.C, reaction, adding water, stirring, regulating pH=6-7, removing lower-layer water, and negative-pressure distilling to remove toluene and obtain product.
Owner:陈克付
Preparation method of octyl (R)-2-(4-chloro-2-methylphenoxy) propionate root retarder
ActiveCN107473962AHigh yieldLittle loss of optical activityPreparation by ester-hydroxy reactionOrganic compound preparationEthyl fumarateRaw material
The invention discloses a preparation method of octyl (R)-2-(4-chloro-2-methylphenoxy) propionate which can be used as a root retarder. The preparation method comprises the following steps: with ethyl L-lactate as a raw material, sulfonating together with p-toluenesulfonyl chloride to obtain a corresponding sulfonyl ester compound; etherifying the sulfonyl ester compound and 4-chloro-o-cresol to obtain a corresponding aromatic ether ester compound; and finally, performing transesterification on the ether ester compound and n-octyl alcohol to obtain octyl (R)-2-(4-chloro-2-methylphenoxy) propionate. Octyl (R)-2-(4-chloro-2-methylphenoxy) propionate prepared by the preparation method provided by the invention is high in optical content, relatively small in optical loss of raw materials and relatively high in yield. The invention establishes a reaction system with the characteristics of simple preparation process, mild reaction condition and low cost, so that the problems that the compound is imported and has a scarce source are solved to a certain extent.
Owner:KESHUN WATERPROOF TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

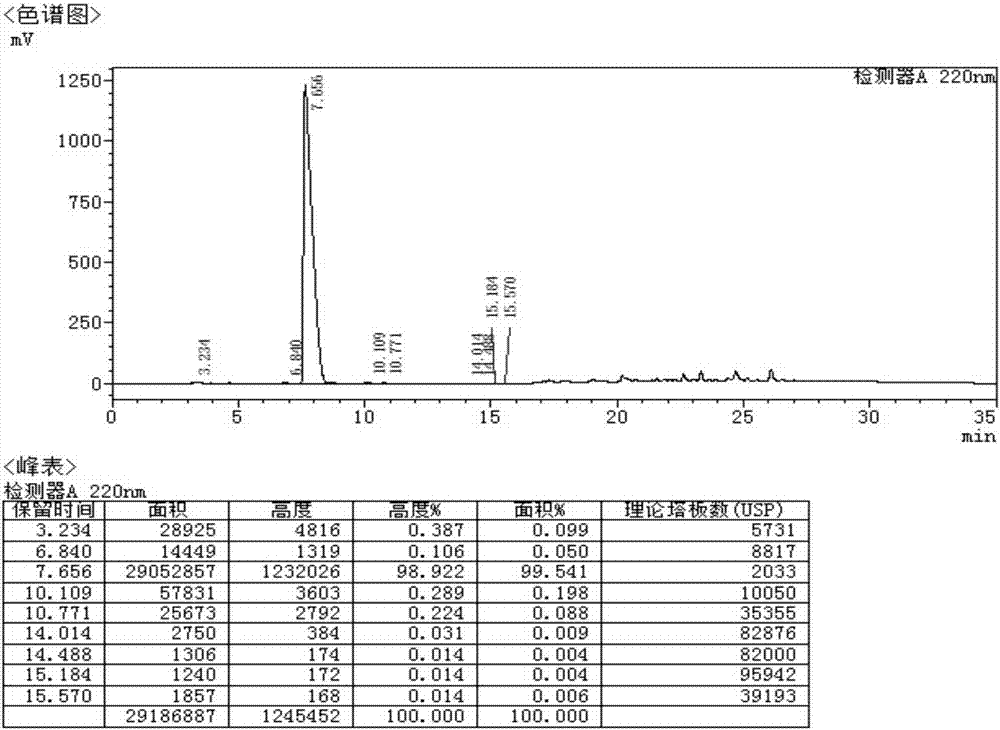
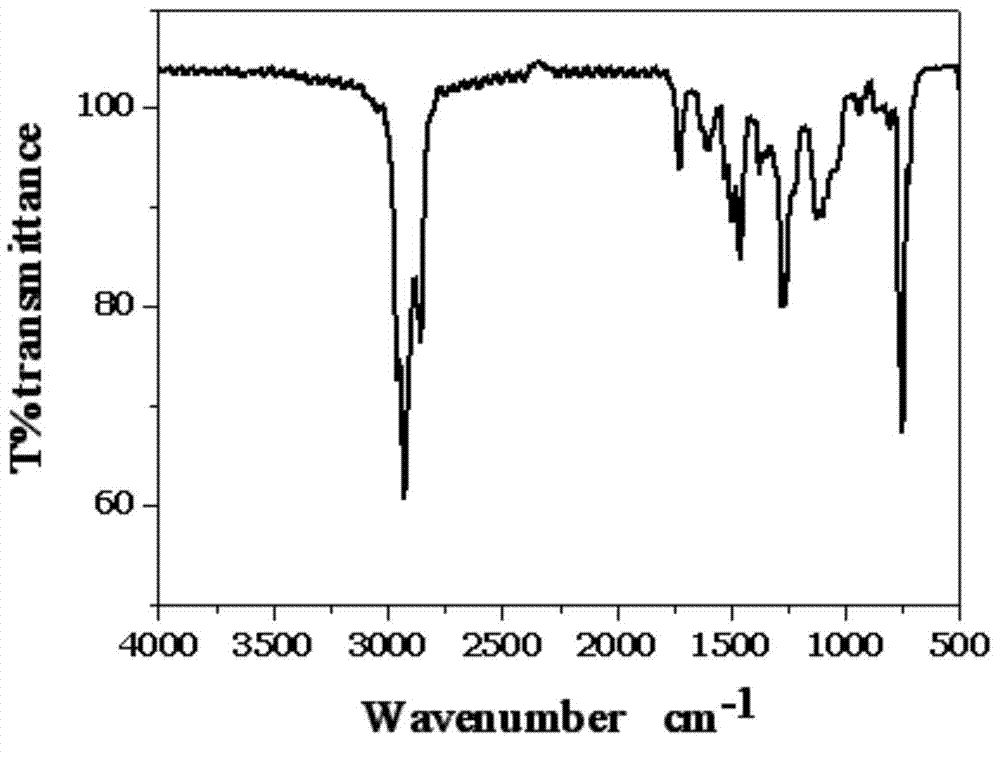
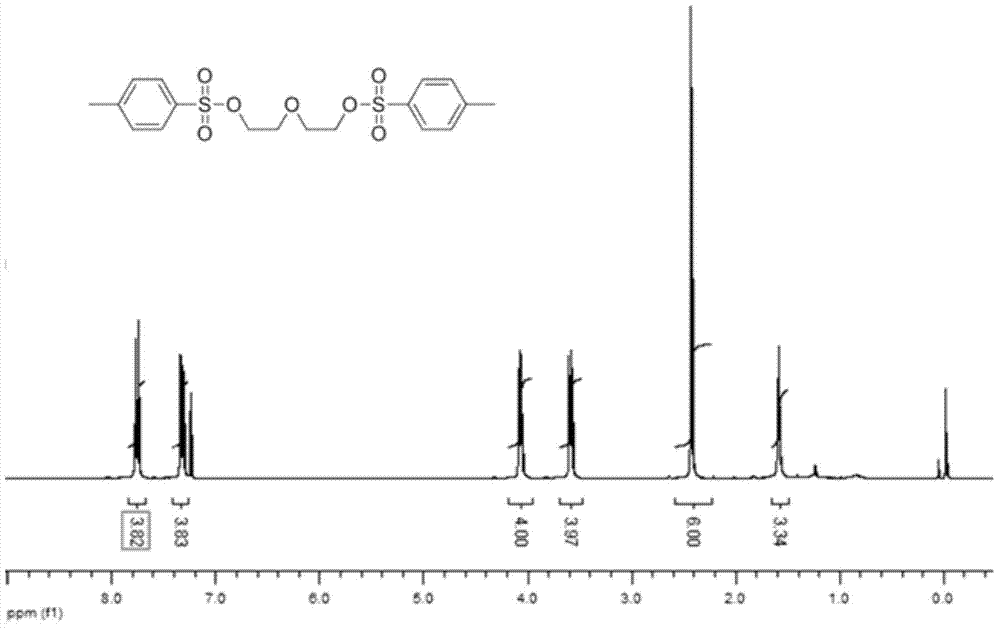
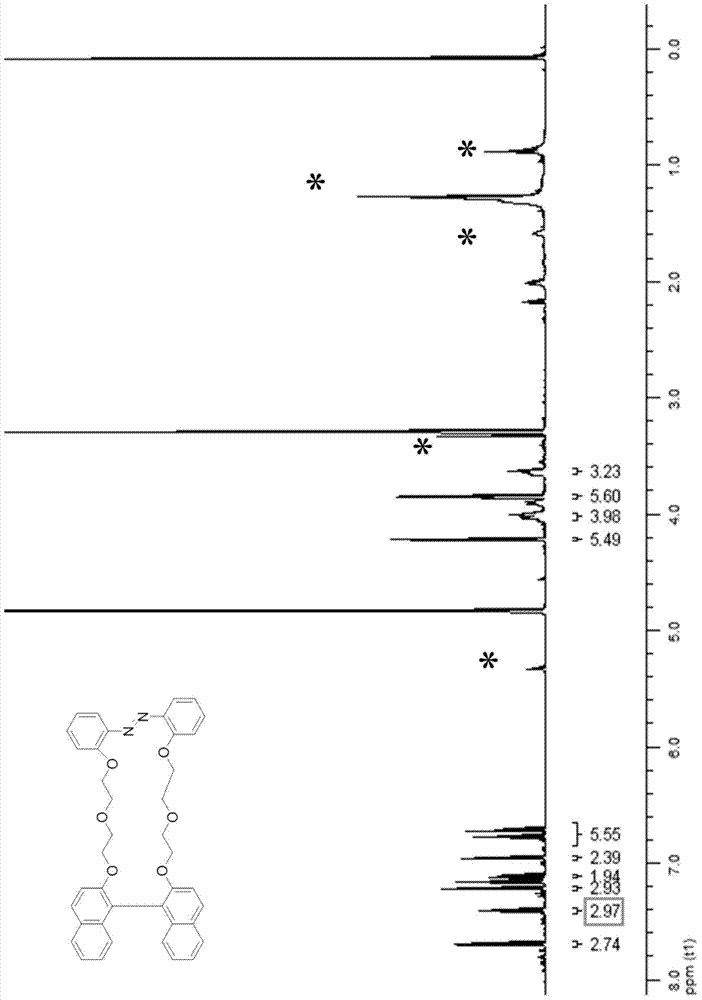
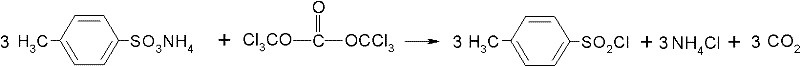
![5,10-dihydroindolo[3,2-b]indole derivative synthesis method 5,10-dihydroindolo[3,2-b]indole derivative synthesis method](https://images-eureka.patsnap.com/patent_img/926a1d87-8ac7-4d56-92b9-60e6b3543e8a/BDA0000987347520000011.PNG)
![5,10-dihydroindolo[3,2-b]indole derivative synthesis method 5,10-dihydroindolo[3,2-b]indole derivative synthesis method](https://images-eureka.patsnap.com/patent_img/926a1d87-8ac7-4d56-92b9-60e6b3543e8a/BDA0000987347520000021.PNG)
![5,10-dihydroindolo[3,2-b]indole derivative synthesis method 5,10-dihydroindolo[3,2-b]indole derivative synthesis method](https://images-eureka.patsnap.com/patent_img/926a1d87-8ac7-4d56-92b9-60e6b3543e8a/FDA0000987347510000011.PNG)