Novel method for synthesis of (S)-propisochlor
A technology of metolachlor and a new method, applied in the new field of synthesizing (S)-metolachlor, achieving the effects of high e.e. value, mild reaction, and overcoming racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
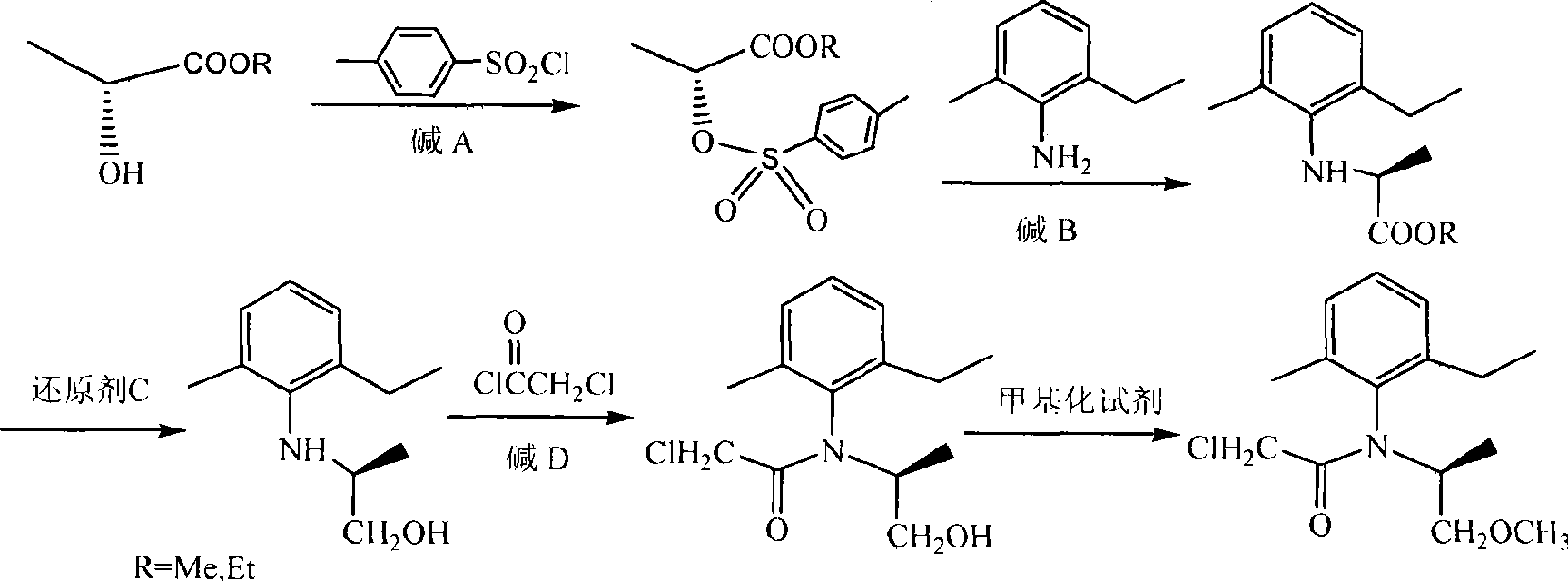
[0014] In a 250 ml three-necked flask equipped with a stirrer and a thermometer, 125 ml of toluene was added, and then 21 grams (0.11 moles) of p-toluenesulfonyl chloride and 10.4 grams (0.1 moles) of (D)-methyl lactate were dropped into the reaction flask 15.2 g (0.15 mol) of triethylamine was added dropwise, the temperature was controlled in an ice-water bath at -10°C to -5°C, and the reaction was tracked until the reaction of (D)-methyl lactate was complete. Filtrate, wash the filtrate with 45 ml of water, separate layers, extract the aqueous layer with 45 ml of dichloromethane, combine the organic layers, dry with 20 g of anhydrous sodium sulfate, filter, and spin the solvent to obtain a light yellow crude product. Then recrystallized with ether:n-hexane=1:1 to obtain 22 g of colorless crystal (R)-2-(p-toluenesulfonyloxy)methyl propionate, with a yield of 85% and an e.e. value of 97.1%. 14.1 grams (0.104 moles) of 2-methyl-6-ethylaniline are dropped into 100 milliliters of...
Embodiment 2
[0019]In a 250 ml three-necked flask equipped with a stirrer and a thermometer, 125 ml of methylene chloride was added, and then 21 grams (0.11 moles) of p-toluenesulfonyl chloride and 10.4 grams (0.1 moles) of (D)-methyl lactate were dropped into 12.1 g (0.12 mol) of triethylamine was added dropwise into the reaction flask, the temperature was controlled in an ice-water bath at -10°C to -5°C, and the reaction was tracked until the reaction of (D)-methyl lactate was complete. Filtrate, wash the filtrate with 45 ml of water, separate layers, extract the aqueous layer with 45 ml of dichloromethane, combine the organic layers, dry with 20 g of anhydrous sodium sulfate, filter, and spin the solvent to obtain a light yellow crude product. Then recrystallized with ether:n-hexane=1:1 to obtain 21.7 g of colorless crystal (R)-2-(p-toluenesulfonyloxy)methyl propionate, with a yield of 84% and an e.e. value of 98%. 11.9 grams (0.088 moles) of 2-methyl-6-ethylaniline were dropped into a ...
Embodiment 3
[0024] In a 250 ml three-necked flask equipped with a stirrer and a thermometer, 125 ml of 1,2-dichloroethane was added, and then 15.3 grams (0.08 moles) of p-toluenesulfonyl chloride and 10.4 grams (D)-methyl lactate ( 0.1 mol) into the reaction flask, drop 12.1 g (0.12 mol) of triethylamine, and control the temperature in an ice-water bath at -10°C to -5°C. The sulfonyl chloride reacts completely. Filtrate, wash the filtrate with 45 ml of water, separate layers, extract the aqueous layer with 45 ml of dichloromethane, combine the organic layers, dry with 20 g of anhydrous sodium sulfate, filter, and spin the solvent to obtain a light yellow crude product. Then recrystallized with ether:n-hexane=1:1 to obtain 17 g of colorless crystal (R)-2-(p-toluenesulfonyloxy)methyl propionate, with a yield of 82% and an e.e. value of 94%. Put 7.2 grams (0.053 moles) of 2-methyl-6-ethylaniline in a 100 ml three-necked flask equipped with a stirrer and a thermometer, and (R)-2-(p-toluenesu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com