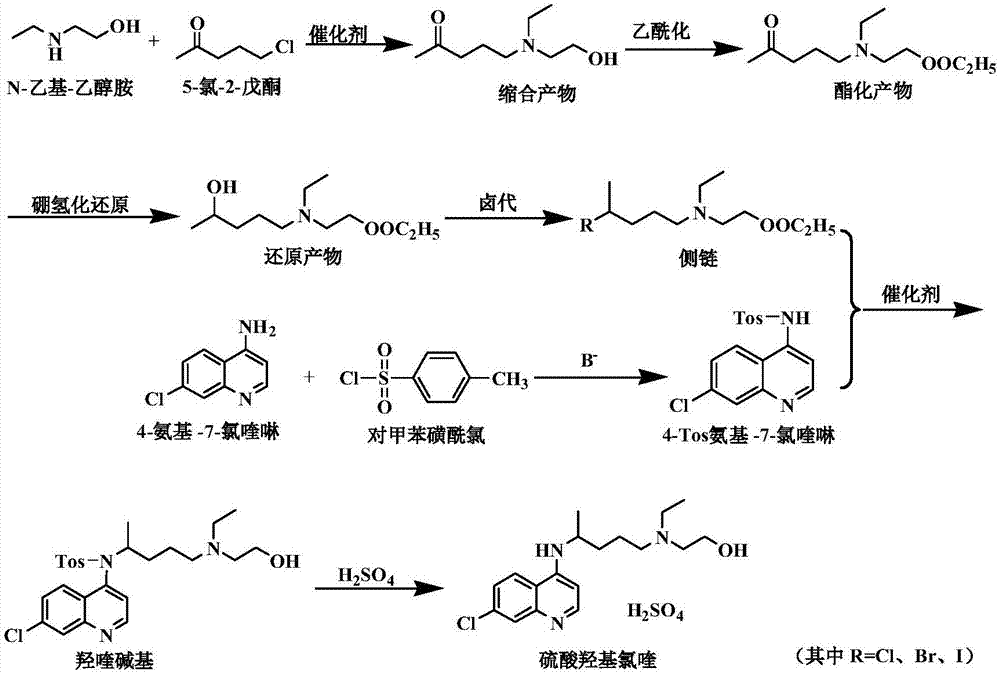
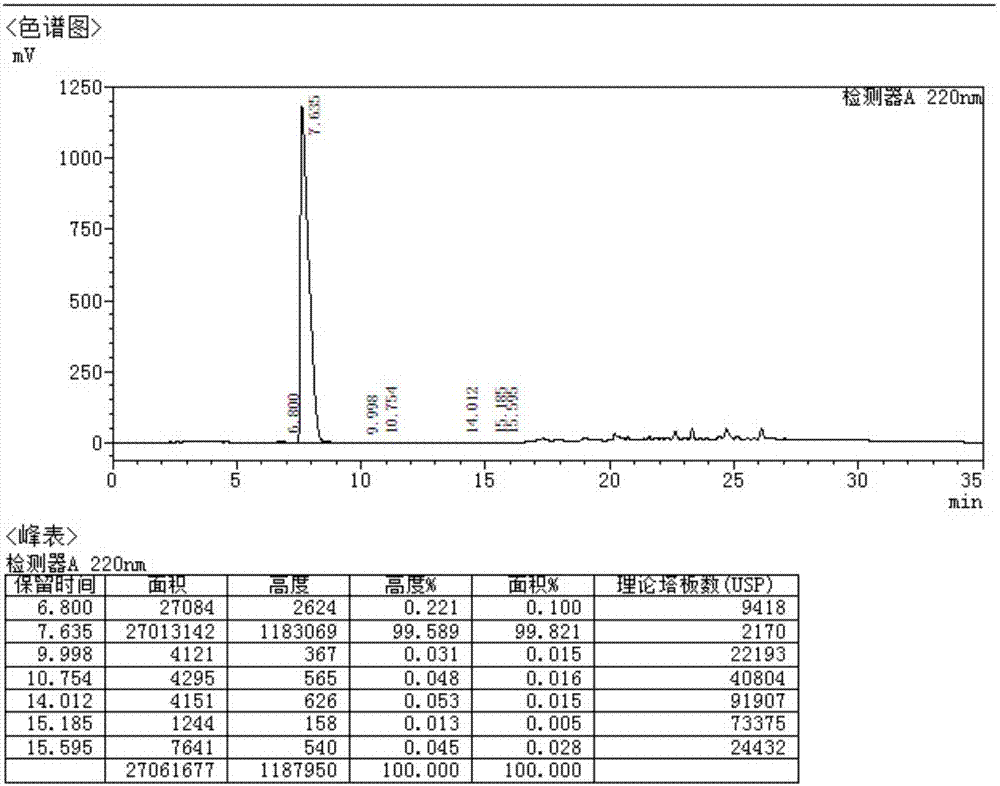
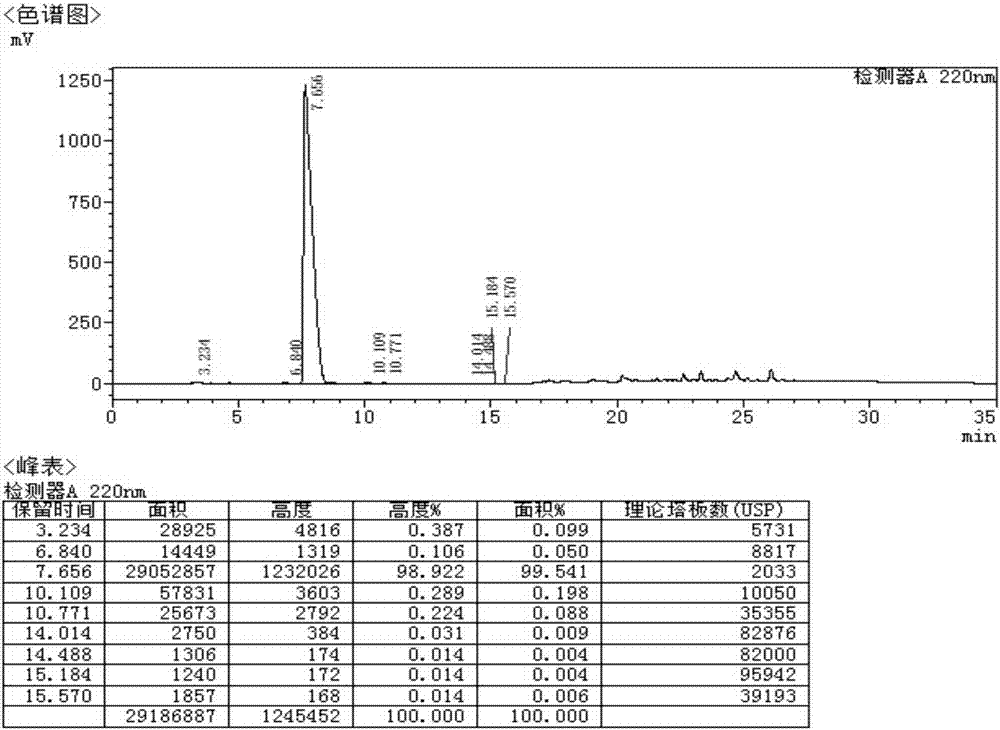
Side chain, synthesis method thereof, and method for synthesizing hydroxychloroquine sulfate from side chain
A technology of hydroxychloroquine sulfate and a synthesis method, applied in the field of medicine and chemical industry, can solve the problems of high safety risk, high impurity content and high raw material cost, and achieve the effects of low process temperature, high yield and quality, and low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Preparation of side chains:
[0106] (1) Condensation reaction: Add 30g of N-ethylethanolamine, 0.6g of tetrabutylammonium bromide, 25g of potassium hydroxide, 240g of chloroform, and 120g of water into the reaction flask, then control the temperature at 20-30°C, and add 38g of 5 -Chloro-2-pentanone, after the dropwise addition, stirred and reacted for 3 hours, static layered, discarded the water phase, added 24g of anhydrous sodium sulfate to the organic layer, controlled the temperature at 10-20°C, stirred and dried for 1 hour, filtered, The filtrate was the organic phase of the condensation product, and its content was detected. The condensation product was 50.2 g, the molar yield was 92% (based on 5-chloro-2-pentanone), and the GC purity was ≧98.5%.
[0107] (2) Esterification reaction: Cool the organic phase of the condensation product (condensation product 50.2g) prepared in step (1) to 0-5°C, and add 24g of acetyl chloride dropwise at heat preservation. After co...
Embodiment 2
[0111] Preparation of side chains:
[0112] (1) Condensation reaction: Add 30g of N-ethylethanolamine, 0.9g of tetrabutylammonium bromide, 25g of potassium hydroxide, 240g of chloroform, and 120g of water into the reaction flask, then control the temperature at 20-30°C, and add 38g of 5 -Chloro-2-pentanone, after the dropwise addition, stirred and reacted for 3 hours, static layered, discarded the water phase, added 24g of anhydrous sodium sulfate to the organic layer, controlled the temperature at 10-20°C, stirred and dried for 1 hour, filtered, The filtrate was the organic phase of the condensation product, and its content was detected. The condensation product was 50.8 g, the molar yield was 93.1% (based on 5-chloro-2-pentanone), and the GC purity was ≧98.5%.
[0113] (2) Esterification reaction: cool down the organic phase of the condensation product (containing 50.8 g of condensation product) prepared in step (1) to 0-5° C., add 24 g of acetyl chloride dropwise while keep...
Embodiment 3
[0117] Preparation of side chains:
[0118] (1) Condensation reaction: Add 30g of N-ethylethanolamine, 1.2g of tetrabutylammonium bromide, 25g of potassium hydroxide, 240g of chloroform, and 120g of water into the reaction flask, then control the temperature at 20-30°C, and add 38g of 5 -Chloro-2-pentanone, after the dropwise addition, stirred and reacted for 3 hours, static layered, discarded the water phase, added 24g of anhydrous sodium sulfate to the organic layer, controlled the temperature at 10-20°C, stirred and dried for 1 hour, filtered, The filtrate was the organic phase of the condensation product, and its content was detected. The condensation product was 51.7g, the molar yield was 94.7% (based on 5-chloro-2-pentanone), and the GC purity was ≧98.5%.
[0119] (2) Esterification reaction: Cool the organic phase of the condensation product (condensation product 51.7g) prepared in step (1) to 0-5°C, and add 24g of acetyl chloride dropwise at heat preservation. After c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com