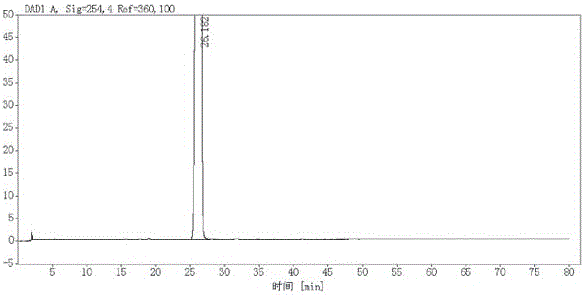
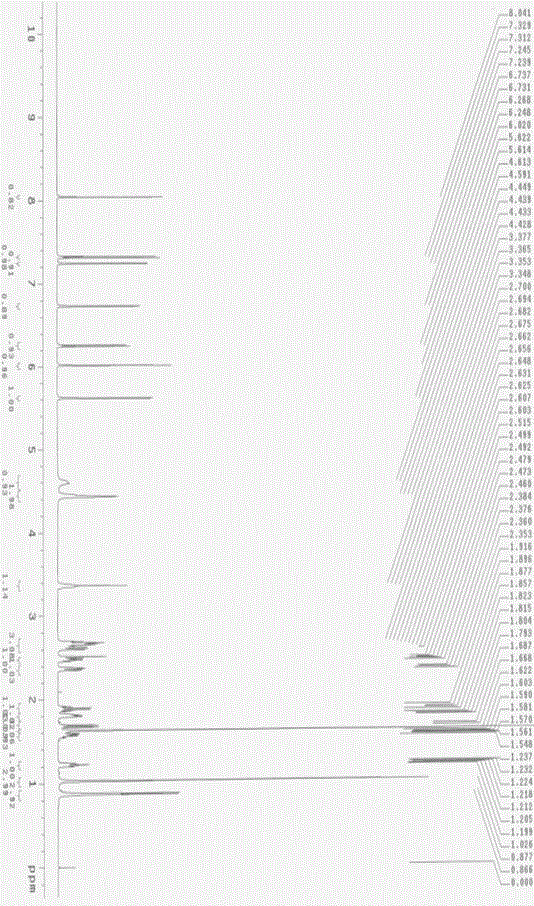
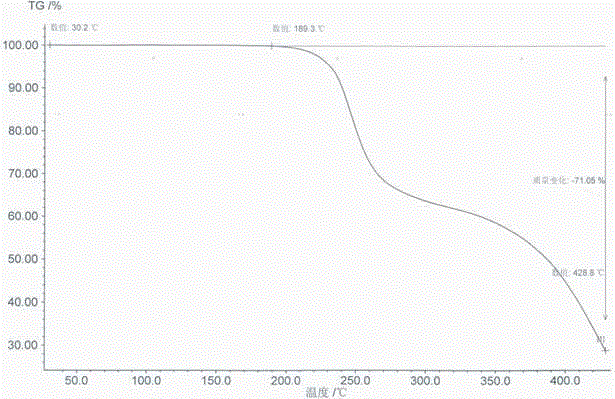
Method for synthesizing mometasone furoate or monohydrate of mometasone furoate
A technology of mometasone furoate and monohydrate, which is applied in the field of drug synthesis to achieve the effects of high utilization rate, increased reaction yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Preparation of mometasone furoate crude product
[0052] Add 300ml of dichloromethane, 50g of 8-DM, and 30.7g of p-toluenesulfonyl chloride to the reaction bottle, cool the reaction solution to -5~5°C, add 45ml of triethylamine dropwise, and react at -5~5°C for 9 hours. TLC detects that the reaction is complete (using the dichloromethane solution of compound 1 as a reference solution, taking a small amount of reaction solution as a test solution, spotting on a GF254 silica gel plate, developing with a mixed solvent with a volume ratio of dichloromethane:methanol=20:1, Check under 254nm ultraviolet light, need test solution not to contain the spot of control solution, be reaction completion); After reaction is finished, reaction solution contains compound 2; Then add triethylamine hydrochloride 147g in above-mentioned reaction solution, be warming up to reflux, Reacted for 3 hours, TLC monitored the completion of the reaction (with the dichloromethane solution of com...
Embodiment 2
[0057] (1) Preparation of mometasone furoate crude product
[0058] Add 300ml of dichloromethane, 50g of 8-DM, and 30.7g of p-toluenesulfonyl chloride to the reaction bottle, cool the reaction solution to -5~5°C, add 45ml of triethylamine dropwise, and react at -5~5°C for 9 hours. TLC detects that the reaction is complete (using the dichloromethane solution of compound 1 as a reference solution, taking a small amount of reaction solution as a test solution, spotting on a GF254 silica gel plate, developing with a mixed solvent with a volume ratio of dichloromethane:methanol=20:1, Check under the 254nm ultraviolet lamp, the test solution does not contain the spots of the control solution, which means that the reaction is complete); after the reaction is completed, add lithium chloride 45.5g, heat up to reflux, react for 3h, and TLC monitors that the reaction is complete (with the dichloride of compound 2 The methane solution was used as the control solution, and a small amount o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com