Thiophene polycyclic organic semiconductor material synthesis based on pyrene
An organic semiconductor, thiophene technology, applied in the field of photoelectric conversion materials, can solve the problem of non-fluorescence and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
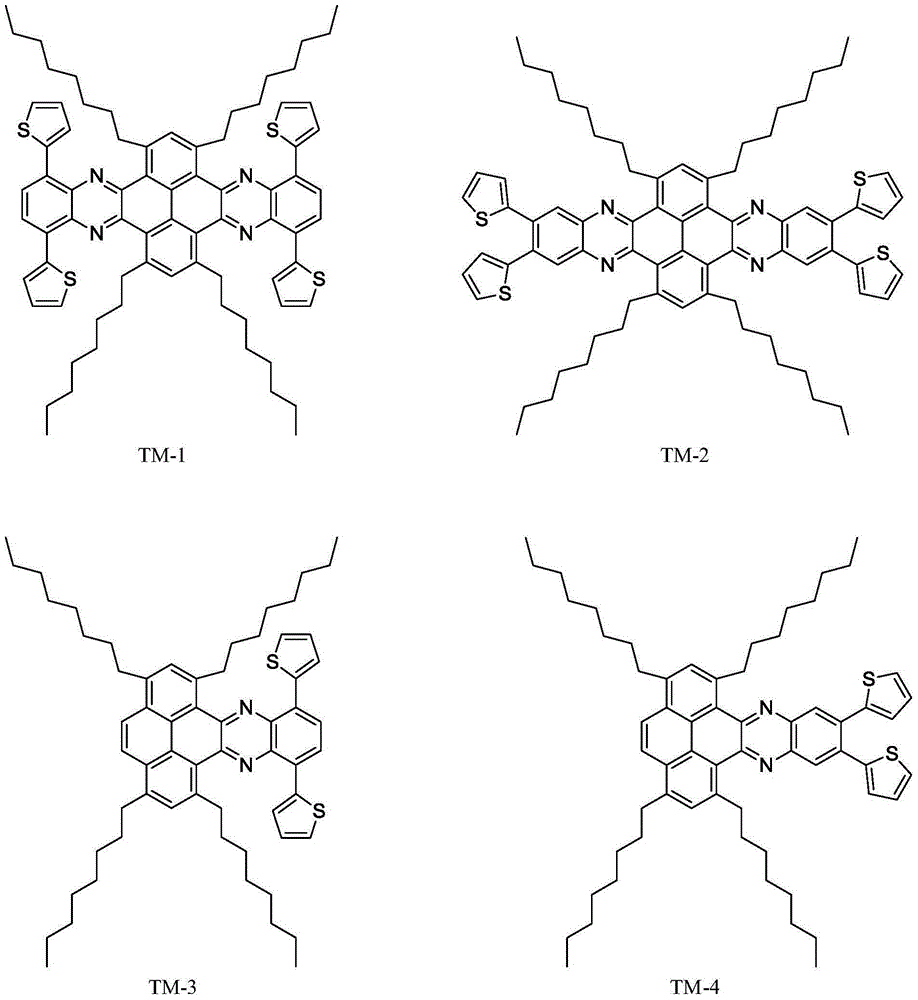
[0062] The preparation method of thiophene polycyclic organic semiconductor material compound TM-1 based on pyrene, comprises the steps:
[0063] (1) Synthesis of 1,3,6,8-tetrabromopyrene
[0064] Add 6g of pyrene and 100mL of nitrobenzene to a 250mL three-necked round-bottom flask in sequence, and add 6.8mL of liquid bromine using a syringe while stirring at room temperature; heat, stir and reflux at 220°C for 4 hours; Wash and dry overnight in vacuum to obtain a gray-green solid that is 1,3,6,8-tetrabromopyrene;
[0065] (2) Synthesis of 1,3,6,8-tetraoctynepyrene
[0066] Add 6.12g of 1,3,6,8-tetrabromopyrene, 120mL of dry tetrahydrofuran and 120mL of diisopropylamine to a 500mL three-necked round-bottomed flask; then vacuumize, fill with nitrogen twice, and add 252mg of Cuprous iodide and 852mg bis(triphenylphosphine)palladium dichloride, vacuumize and fill with nitrogen twice; then inject 12mL of 1-octyne with a syringe under stirring conditions, vacuumize and fill with ...
Embodiment 2
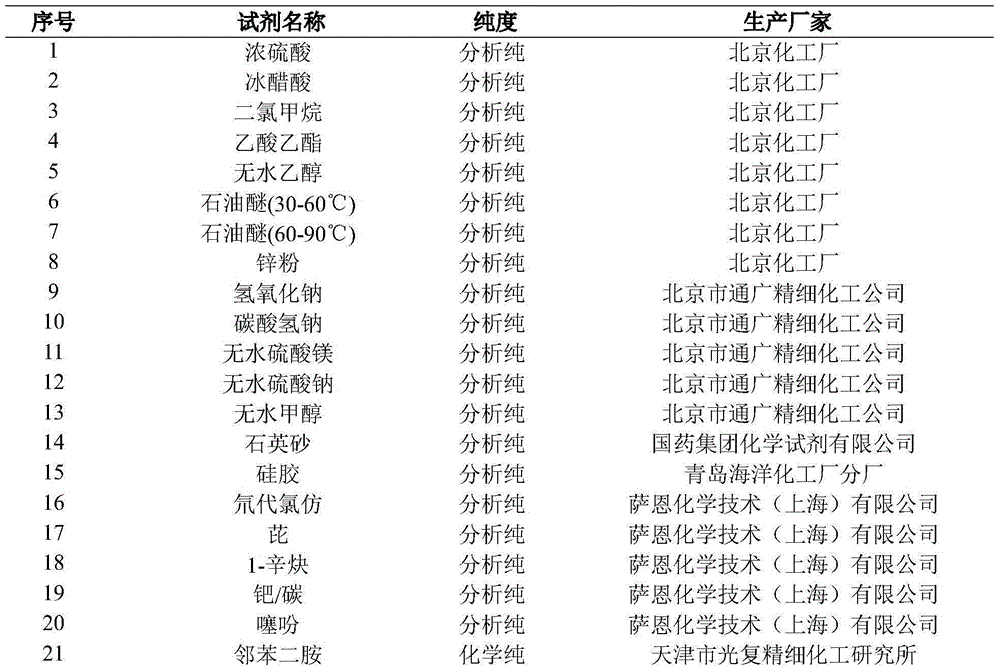
[0082] The preparation method of thiophene polycyclic organic semiconductor material compound TM-2 based on pyrene, comprises the steps:
[0083] (1) Synthesis of 1,3,6,8-tetrabromopyrene
[0084] Add 6g of pyrene and 100mL of nitrobenzene to a 250mL three-necked round-bottom flask in sequence, and add 6.8mL of liquid bromine using a syringe while stirring at room temperature; heat, stir and reflux at 220°C for 4 hours; Wash and dry overnight in vacuum to obtain a gray-green solid that is 1,3,6,8-tetrabromopyrene;
[0085] (2) Synthesis of 1,3,6,8-tetraoctynepyrene
[0086] Add 6.12g of 1,3,6,8-tetrabromopyrene, 120mL of dry tetrahydrofuran and 120mL of diisopropylamine to a 500mL three-necked round-bottomed flask; then vacuumize, fill with nitrogen twice, and add 252mg of Cuprous iodide and 852mg bis(triphenylphosphine)palladium dichloride, vacuumize and fill with nitrogen twice; then inject 12mL of 1-octyne with a syringe under stirring conditions, vacuumize and fill with ...
Embodiment 3
[0106] The preparation method of thiophene polycyclic organic semiconductor material compound TM-3 based on pyrene, comprises the steps:
[0107] (1) Synthesis of 1,3,6,8-tetrabromopyrene
[0108] Add 6g of pyrene and 100mL of nitrobenzene to a 250mL three-necked round-bottom flask in sequence, and add 6.8mL of liquid bromine using a syringe while stirring at room temperature; heat, stir and reflux at 220°C for 4 hours; Wash and dry overnight under vacuum to obtain a gray-green solid that is 1,3,6,8-tetrabromopyrene;
[0109] (2) Synthesis of 1,3,6,8-tetraoctynepyrene
[0110]Add 6.12g of 1,3,6,8-tetrabromopyrene, 120mL of dry tetrahydrofuran and 120mL of diisopropylamine to a 500mL three-necked round-bottomed flask; then vacuumize, fill with nitrogen twice, and add 252mg of Cuprous iodide and 852mg bis(triphenylphosphine)palladium dichloride, vacuumize and fill with nitrogen twice; then inject 12mL of 1-octyne with a syringe under stirring conditions, vacuumize and fill wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com