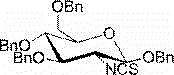
2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose, and synthetic method and application thereof
A technology of isothiocyanate and glucopyranose, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of high risk and low yield, and achieve safe yield and side effects The effect of less product and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1, a 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O-benzyl- β -D-glucopyranose, its molecular formula is as follows:
[0022]
Embodiment 2
[0023] Example 2, a 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O-benzyl- β -D-glucopyranose synthetic method, it is characterized in that, first 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl- β -D-pyranose hydrochloride, carbon disulfide and triethylamine are reacted, and then reacted with p-toluenesulfonyl chloride to obtain 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O- Benzyl- β -D-glucopyranose; reaction with acetonitrile as solvent, 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl- β - The molar ratio of D-pyranose hydrochloride to carbon disulfide, triethylamine and p-toluenesulfonyl chloride is 1:1.2:3.0:1, the reaction temperature is 0°C, and the reaction time is 1 hour.
Embodiment 3
[0024] Example 3, a 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O-benzyl- β -D-glucopyranose synthetic method, it is characterized in that, first 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl- β -D-pyranose hydrochloride, carbon disulfide and triethylamine are reacted, and then reacted with p-toluenesulfonyl chloride to obtain 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O- Benzyl- β -D-glucopyranose; reaction with acetonitrile as solvent, 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl- β - The molar ratio of D-pyranose hydrochloride to carbon disulfide, triethylamine and p-toluenesulfonyl chloride is 1.2:1:3.5:1.2, the reaction temperature is 0°C, and the reaction time is 2 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com