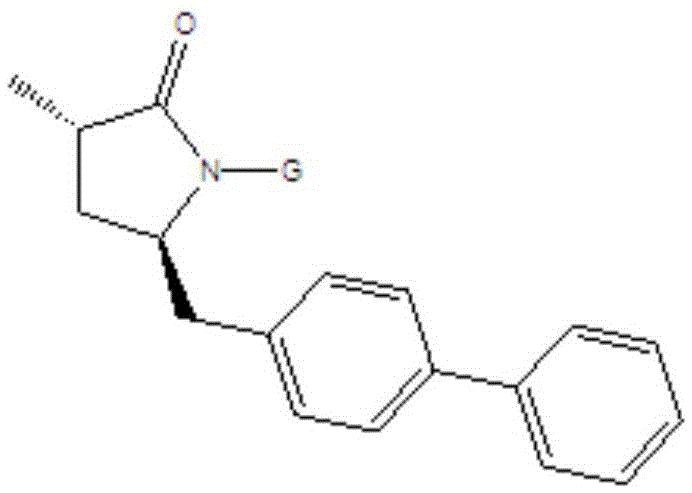
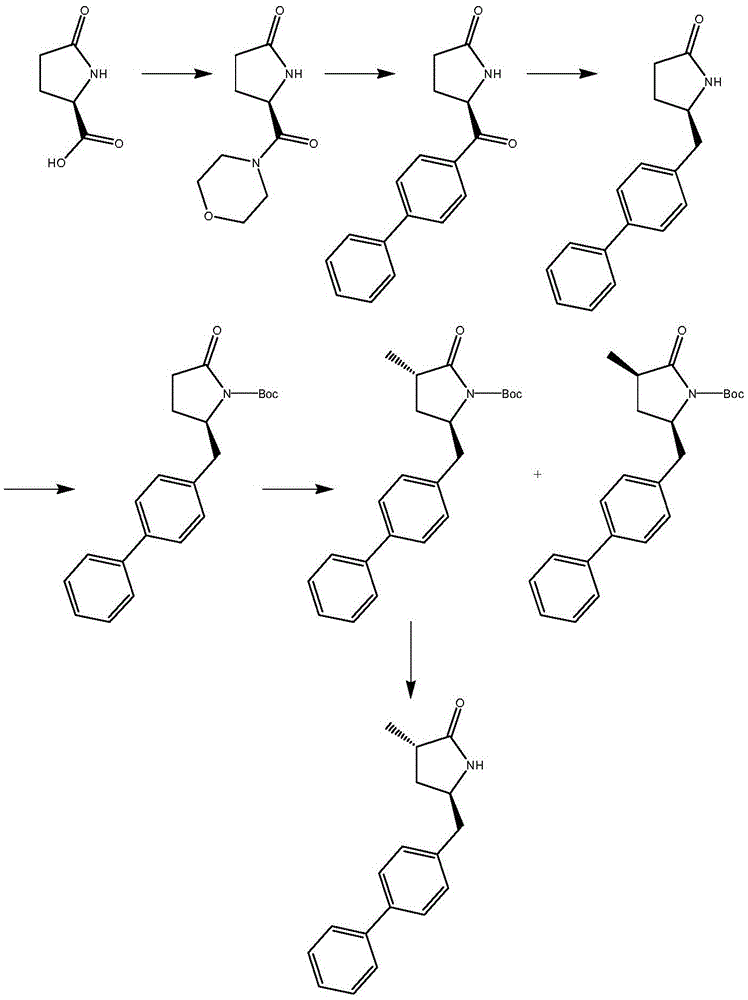
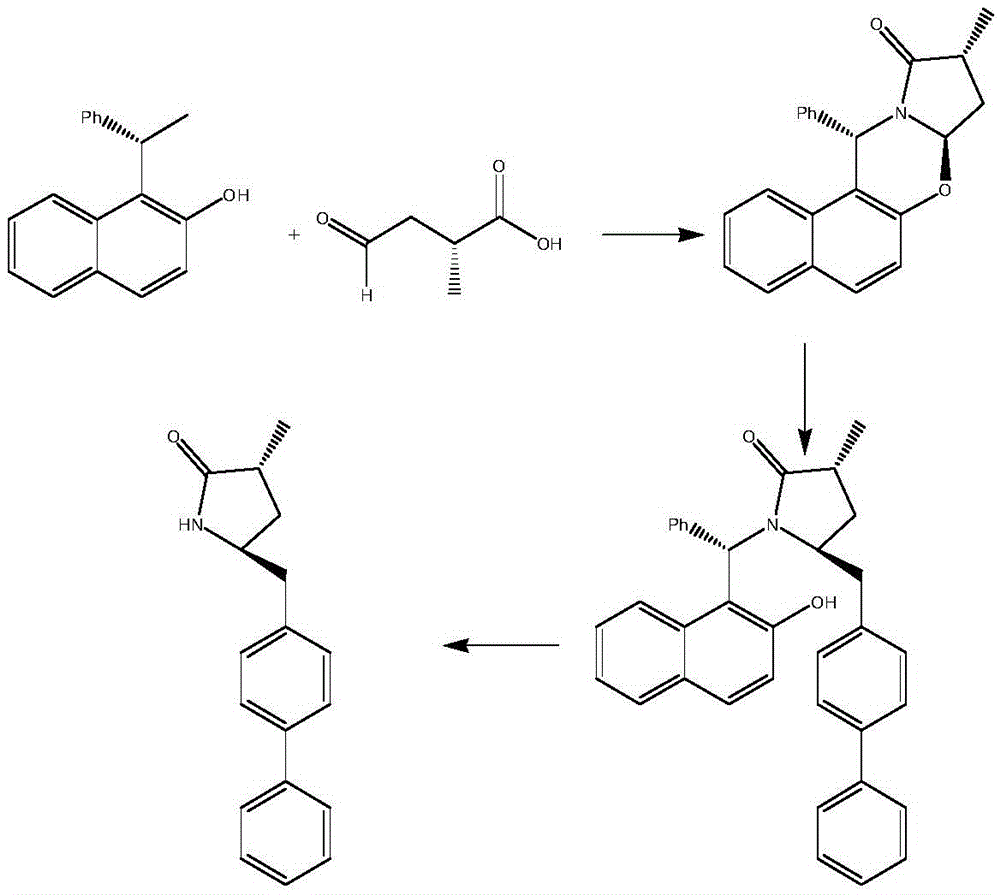
Preparation method for Sacubitril intermediate
An intermediate and methyl technology, applied in the field of organic synthesis route design API intermediate preparation, can solve the problems of inability to apply chiral adjuvant, raw materials are not easy to obtain, cost increase, etc., achieve high purity and yield, and novel method , the effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 2.3g of (3R,5S)-5-(hydroxymethyl)-3-methyl-2-pyrrolidone into a 250ml three-neck flask, then add 100ml of tetrahydrofuran, add 16ml of 10% sodium hydroxide solution under stirring, and put in an ice-water bath Cool, when the internal temperature drops to 5°C, slowly add a solution of 7.76g of p-toluenesulfonyl chloride and 50ml of tetrahydrofuran dropwise, keep the internal temperature below 10°C during the dropwise addition, remove the cooling bath, stir overnight at room temperature, and The reaction solution was poured into 200ml of ice water, adjusted to PH=6-7 with 37% HCl, evaporated to remove tetrahydrofuran, added 250ml of n-hexane, stirred to precipitate a solid, filtered with suction, and dried to obtain 4.5g. Yield: 88.9%
[0046] 1HNMR(DMSO-d6ppm)0.97(d,J=7.2,3H),1.66-1.74(m,1H),1.89-1.94(m,1H),2.25-2.29(m,1H),2.43(s,3H) ,3.6-3.7(m,1H),3.87-3.90(m,1H),3.94-3.99(m,1H),7.48-7.50(d,J=8,2H),7.76(brs,1H),7.79- 7.81 (d,J=8,2H).
Embodiment 2
[0048] Add 2.3g of (3R,5S)-5-(hydroxymethyl)-3-methyl-2-pyrrolidone into a 250ml three-neck flask, then add 100ml of dichloromethane, 5.6ml of triethylamine, and 0.02g of DMAP, ice Cool in a water bath. When the internal temperature drops to 5°C, slowly add a solution of 7.76g of p-toluenesulfonyl chloride and 50ml of dichloromethane dropwise. During the dropwise addition, keep the internal temperature below 10°C. After dropping, remove the cooling bath and stir at room temperature Overnight, the reaction solution was poured into 200ml of ice water, adjusted to PH=6-7 with 37% HCl, separated into layers, the water layer was extracted once with 100ml of dichloromethane, the dichloromethane layers were combined, dried over anhydrous magnesium sulfate, evaporated Remove dichloromethane, recrystallize with ethyl acetate, filter with suction, and dry to obtain 3.1g. Yield: 91.1%
Embodiment 3
[0050] Add 2.72g of (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidone into a 100ml three-neck flask, then add 50ml of acetone and 2.25g of sodium iodide, and heat up and reflux for 24 hours , cooled, evaporated to remove acetone, added 40ml of water, extracted three times with 150ml of ethyl acetate, combined the ethyl acetate layers, dried over anhydrous magnesium sulfate, evaporated to remove ethyl acetate, recrystallized with ethyl acetate, and dried to obtain 2.1g. Yield: 92.1% 1HNMR (DMSO-d6ppm) 1.03 (d, 3H), 1.74-1.82 (m, 1H), 1.96-2.02 (m, 1H), 2.42-2.49 (m, 1H), 3.27-3.28 (m ,2H), 3.58-3.59(m,1H), 7.79(brs,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com