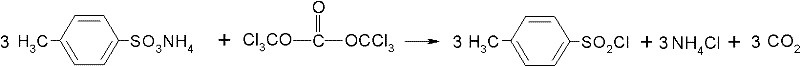Method for preparing paratoluensulfonyl chloride
A technology of p-toluenesulfonyl chloride and ammonium p-toluenesulfonate, which is applied in the field of preparation of p-toluenesulfonyl chloride, can solve problems such as complicated separation steps, unsafe use, and impact on product quality, and achieves convenient and easy-to-obtain raw materials and mild reaction conditions , The effect of simple purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The reactor is a three-necked flask with a volume of 250ml, equipped with a thermometer, mechanical stirring, reflux condenser and drying tube. 18.9 g of ammonium p-toluenesulfonate (100 mmol), 0.73 g of N,N-dimethylformamide (10 mmol) and 50 g of dichloroethane were added to the flask at one time. Stirring was started, and at room temperature, the dichloroethane solution (which contained 10 g triphosgene (34 mmol), 50 g of dichloroethane) dissolved with triphosgene was slowly and uniformly added dropwise to the reaction flask, and the dropping time was 30 min. After adding, keep warm for 30min. After heat preservation, the temperature was raised until obvious reflux phenomenon appeared in the system, and the reaction was maintained under reflux conditions for three hours. After the reaction liquid is cooled, the filter cake ammonium chloride is removed by filtration, and the obtained filtrate is subjected to vacuum distillation operation. After recovering 92g of dichl...
Embodiment 2~7
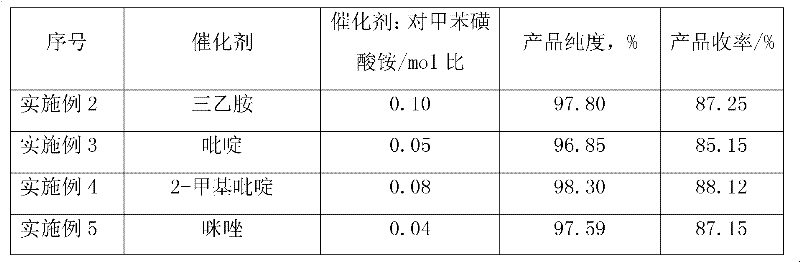
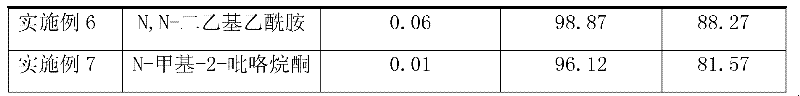
[0031] The type and amount of the organic base catalyst were changed, and other reaction conditions and raw material dosage were the same as in Example 1. The experimental results are shown in Table 1 below:
[0032] Table 1
[0033]
[0034]
[0035]As can be seen from the results in Table 1, the use of different types of organic amines as catalysts can obtain better experimental results, and if the amount of catalyst is large, the yield of p-toluenesulfonyl chloride is slightly higher, but there is no great impact on product purity.
Embodiment 8~13
[0037] Using N,N-diethylacetamide as the organic amine, changing the type and amount of solvent used in the reaction, distilling the filtrate after the reaction to recover 92% of the organic solvent, and then proceeding to the crystallization operation. Other reaction conditions and raw material charging amount are identical with embodiment 1. The experimental results are shown in Table 2 below:
[0038] Table 2
[0039]
[0040] As can be seen from the results in Table 2, the use of different types of inert organic solvents can obtain better experimental results, but when using chloroform as a solvent, the p-toluenesulfonyl chloride crystalline purity obtained is slightly lower, and is obtained in ethyl acetate and The product in petroleum ether has good crystallization performance and the obtained product has high purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com