Method for synthesizing 24-epibrassinolide
A technology of epibrassinolide and lactonization, which is applied in the direction of steroids and organic chemistry, can solve the problems of high toxicity, pollution, and environmental pollution, and achieve mild reaction conditions, short process flow, and low environmental pollution. pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
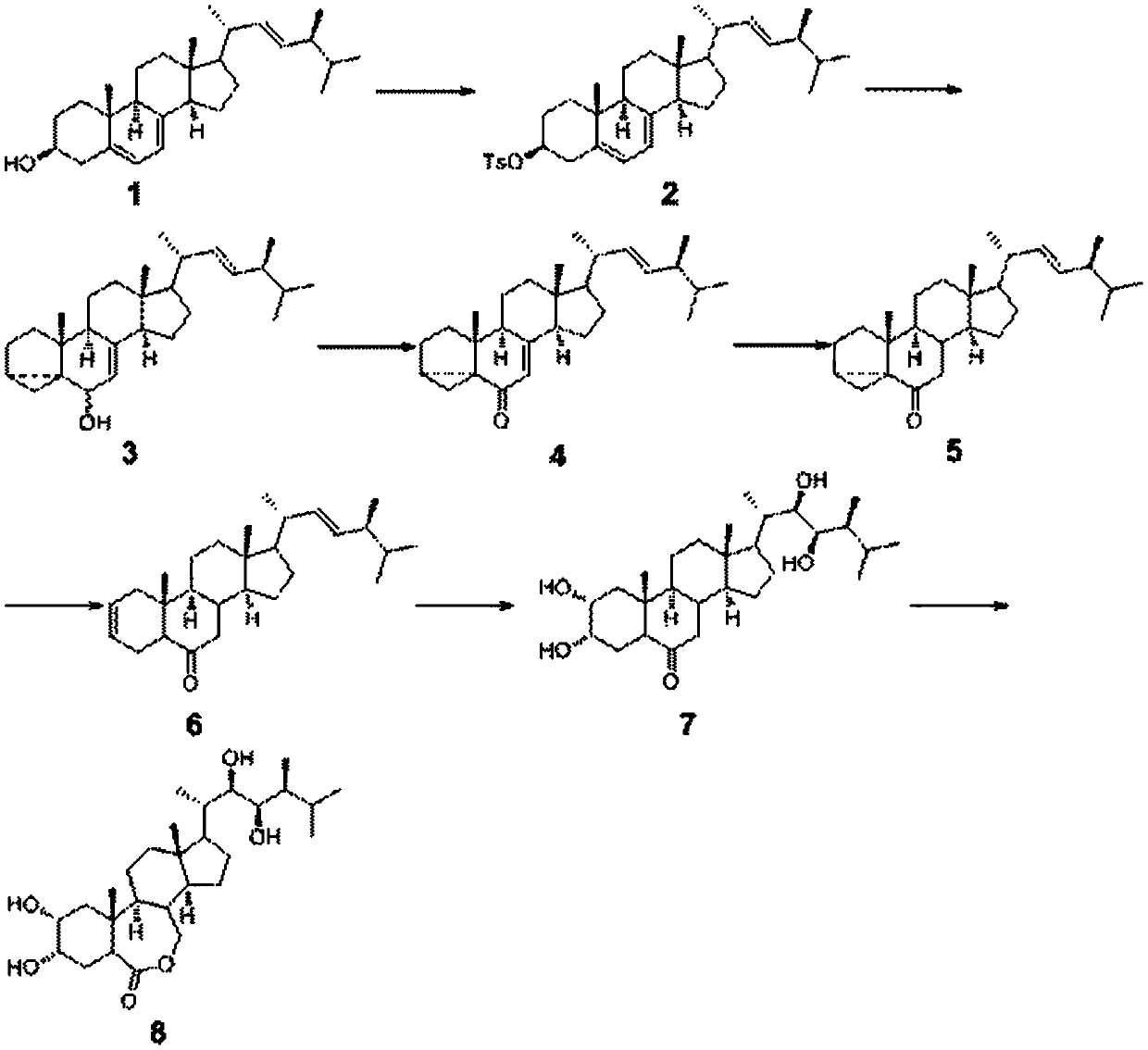
[0024] (22E,24R)--5α-ergosterol-5,7,22-triene-3-p-toluenesulfonyl ester (compound 2) was prepared by putting ergosterol 39.6g (0.1mol) into the four-necked bottle, triethyl 12.1g (0.12mol) of amine, 300ml of toluene, lower the temperature to 0-5°C, add 21.0g (0.11mol) of p-toluenesulfonyl chloride dropwise, and react at 5°C for 2h. After the reaction, filter with suction and wash the filtrate twice with water. Negative pressure precipitation yielded 52.9 g of sulfonylated ergosterol (compound 2), with a yield of 95.9%.
[0025] Preparation of α-5-cyclo-5α-ergosta-7,22-dien-6-ol (compound 3)
[0026] Drop into potassium bicarbonate 9.1g (0.091mol) in the four-necked flask, 300ml water, and add 1200ml acetone, heat up to reflux, then 50g (0.091mol) compound 2 points are added in the four-necked flask in 2 times, after adding, Reflux reaction for 3h. After the reaction was completed, the acetone was distilled off, and then the reaction solution was lowered to room temperature, ...
Embodiment 2
[0041] (22E,24R)--5α-ergosta-5,7,22-triene-3-p-toluenesulfonyl ester (compound 2)
[0042] Put 39.6g (0.1mol) of ergosterol, 20.73g (0.15mol) of potassium carbonate, and 300ml of toluene into the four-necked bottle, cool down to 5-10°C, add 22.9g (0.12mol) of p-toluenesulfonyl chloride dropwise, 8 The reaction was carried out at ℃ for 2 hours. After the reaction was completed, suction filtration was carried out, the filtrate was washed twice with water, and the solvent was precipitated under negative pressure to obtain 52.8 g of sulfonylated ergosterol, with a yield of 94.7%.
[0043] α-5-cyclo-5α-ergosta-7,22-dien-6-one (compound 4):
[0044] Put 330g (0.076mol) of the compound and 100ml of methanol into the four-neck flask, add 19.83g (0.228mol) of manganese dioxide powder regenerated three times and 120ml of water, inject air with an air pump, and raise the temperature to 60°C for 8 hours. After suction filtration, the filtrate was extracted three times with ethyl acetate,...
Embodiment 3
[0049] (22E,24R)--5α-ergosta-5,7,22-triene-3-p-toluenesulfonyl ester (compound 2)
[0050] Put 39.6g (0.1mol) of ergosterol, 13.8g (0.13mol) of sodium carbonate, and 330ml of toluene into the four-necked bottle, cool down to 10°C, add 24.78g (0.13mol) of p-toluenesulfonyl chloride dropwise, and react at 10°C After 2 hours of reaction, suction filtration was performed, the filtrate was washed twice with water, and precipitation under negative pressure was performed to obtain 53.0 g of sulfonylated ergosterol with a yield of 96.3%.
[0051] α-5-cyclo-5α-ergosta-7,22-dien-6-one (Compound 4)
[0052] Put 30g (0.076mol) of compound 3 and 100ml of methanol into the four-neck flask, add 6.61g (0.076mol) of manganese dioxide regenerated six times and 110ml of water, use an air pump to introduce air, and heat up to 20°C for 14h. After suction filtration, the filtrate was extracted three times with ethyl acetate, dried, and concentrated to obtain 28.3 g of compound 4 with a yield of 93...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com